小麦是世界上种植面积最广泛的粮食作物,全球约有2.17亿hm2土地种植小麦,产量仅次于玉米和稻谷。小麦在种植和贮藏过程中易受到镰刀菌属、曲霉属、青霉属、链格孢菌属和麦角菌属等真菌的侵染。这些真菌会产生次生代谢产物,如镰刀菌毒素、黄曲霉毒素(aflatoxins,AFs)和赭曲霉毒素A(ochratoxin A,OTA)等,对人类健康和农业经济造成严重危害。小麦中常见的镰刀菌毒素包括单端孢霉烯族化合物(trichothecenes,TCTs)、玉米赤霉烯酮(zearalenone,ZEN)和伏马毒素B类(fumonisins B,FBs)。其中TCTs又分为A型TCTs,包括T-2毒素、HT-2毒素、新茄病镰刀菌烯醇(neosolaniol,NEO)、蛇形菌素(diacetoxyscirpenol,DAS);B型TCTs,包括脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON)及其衍生物3-乙酰脱氧雪腐镰刀菌烯醇(3-acetyldeoxynivalenol,3-Ac-DON)、15-乙酰脱氧雪腐镰刀菌烯醇(15-acetyldeoxynivalenol,15-Ac-DON)、雪腐镰刀菌烯酮(nivaleno,NIV)和镰刀菌烯酮(fusarenon X,FUS-X)。这些都是污染小麦的真菌毒素,能通过生物链在人体内富集,危害健康。近些年来,一些被称为新兴的真菌毒素也逐渐受到关注,如恩镰孢菌素(enniatins,ENNs)、白僵菌素(beauvericin,BEA)、大镰刀孢菌素(culmorin,CUL)和串珠镰刀菌素(moniliformin,MON);链格孢霉毒素如交链孢酚(alternariol,AOH)、交链格孢酚单甲醚(alternariol monomethyl ether,AME)、细交链格孢菌酮酸(tenuazonic acid,TeA)、腾毒素(tentoxin,TEN)、交链孢烯(altenuene,ALT)、交链孢毒素(altertoxins-Ⅰ/Ⅱ/Ⅲ,ATX-Ⅰ/Ⅱ/Ⅲ);棒曲霉素(patulin,PAT)以及麦角生物碱(ergot alkaloids,EAs)等。
本文汇总了国内外近几年小麦和小麦粉中真菌毒素的污染数据,分析了小麦中主要真菌毒素污染规律,介绍了多种真菌毒素色谱同时检测技术,并对比分析了各种基于色谱检测技术的优点和局限性,以期为小麦中真菌毒素防控和同时检测提供参考。
1 小麦中真菌毒素污染现状
小麦中真菌毒素来源为:田间生长真菌感染小麦植株、收获后贮运过程中真菌感染、收获前温暖潮湿的气候以及收获后不当的贮运条件。因此,及时掌握真菌毒素污染现状和发生规律,加强对主要毒素的监管,对于保障小麦及其制品安全具有积极影响。
1.1 国内污染现状
1.1.1 国内小麦中真菌毒素污染现状
近年来,镰刀菌属仍然是小麦中真菌毒素污染的主要来源,真菌毒素污染呈现出多种毒素共同检出,污染水平年份间差异较大的特点(表1)。调查显示,B型TCTs是小麦中最常见污染物,DON和3-AcDON是其主要污染物,2021年八省小麦DON检出率高达95%以上。DON的主要衍生物3-Ac-DON、15-Ac-DON与DON-3-葡萄糖苷(DON-3-glucoside,DON-3G)阳性率与污染水平相对较低,但毒性与DON相似,能在人体内能重新转化生成DON,诱导增加DON总毒性[9]。目前,国内关于DON-3G污染状况的报道较少。研究发现种植区域是影响DON-3G污染浓度最重要因素,且DON-3G与DON之间存在正相关趋势[10]。贮藏期间DON-3G和DON含量总体呈显著负相关,但应激产毒期二者含量均上升[11]。小麦中DON的污染与田间赤霉病流行程度紧密相关,控制赤霉病发生是控制小麦中DON含量的最好方法。
表1 国内小麦中真菌毒素污染现状
Table 1 Current status of mycotoxin contamination in domestic wheat

地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献DON95.6ND~8 116921.8a[1]ZEN53.6ND~22019.7aBEA11.8ND~27.02.44aENA33.6ND~9.261.11a浙江、江苏、安徽、湖北、河南、ENA136.4ND~68.29.87a山东、山西、河北2021321HPLC-MS/MSENB75.7ND~13610.8aENB155.1ND~19019.9aAME67.0ND~71.64.89aAOH62.3ND~1028.72aTeA1003.0~2 034331.9aTEN99.4ND~13337.0aALT7.17ND~6.172.05a中国201919ICAZEN36.8ND~117.527.1a[2]DON95.5ND~981.9135.9a[3]3-Ac-DON36.7ND~15.25.2a15-Ac-DON37.4ND~29.34.4aNIV34.5ND~89.313.2aZEN8.7ND~57.66.2a河南、河北、安徽、山东2019289HPLC-MS/MSFB115.9ND~21810.6aFB26.2ND~5.04.1aFB37.6ND~6.75.2aAFG10.4ND~1.51.5aAFG21.0ND~0.40.3aAFB14.5ND~2.21.2a
续表1

地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献AFB28.7ND~0.80.4aDON81.67ND~1 002.20104.74[4]3-Ac-DON39.17ND~41.183.7615-Ac-DON0.83ND~5.960.20DON-3G38.33ND~54.715.32ZEN28.33ND~164.998.93OTA———OTB5.00ND~0.510.11OTC23.33ND~3.220.25安徽、江苏、浙江、上海2019120UPLC-MS/MSFB147.50ND~1 700.0622.27FB243.33ND~277.195.25FB340.00ND~292.786.01AFB11.67ND~1.620.12AFB26.67ND~0.620.17T-20.83ND~3.010.17FUS-X4.17ND~55.902.44TeA1004.36~371.8091.19TEN1001.30~57.8420.50ALT1003.81~134.0631.27DON50.8ND~876.0—[5]3-Ac-DON2.1ND~39.2—15-Ac-DON5.0ND~248.8—NIV———河北2017—2019240HPLC-MS/MSZEN0.8ND~323.2—OTA———FB12.5ND~3 889.9—FB20.4ND~1 292.7—FB30.4ND~598.2—DON90.8ND~59 278.02628.5a[6]3-Ac-DON69.2ND~13 109.0135.8a15-Ac-DON49.4ND~804.950.2aNIV45.3ND~3 043.6266.4aZEN27.8ND~22 572.1555.1a安徽、河北、湖北、江苏2018338HPLC- MS/MSFB138.8ND~691.971.8aFB218.6ND~130.834.2aFB325.7ND~125.546.5aAFG1———AFG20.3ND~1.31.3aAFB13.3ND~19.75.0aAFB22.7ND~3.41.7aDON90.3ND~59 278.02 706.3a[7]3-Ac-DON68.7ND~66780.4a15-Ac-DON49.9ND~804.649.6aNIV45.0ND~3 043.6270.4a湖北、安徽、江苏、河北2018371UPLC-MS/MSZEN25.6ND~22 572.1575.9aAFG1———AFG20.3ND~1.31.3aAFB13.2ND~19.74.8aAFB23.2ND~3.51.9a天津201853UPLC-MS/MSBEA69.8ND~77658.4a[8]ENA17.0ND~6.401.83aENA13.8ND~0.380.33aENB30.2ND~12912.4aENB139.6ND~19324.3a
注:HPLC-MS/MS:高效液相色谱-串联质谱法(high performance liquid chromatography-tandem mass spectrometry);UPLC-MS/MS:超高效液相色谱-串联质谱法(ultra performance liquid chromatography tandem mass spectrometry);ICA:免疫层析法(immunochromatography assay);ENA:恩镰孢菌素A(enniatin A);ENA1:恩镰孢菌素A1(enniatin A1);ENB:恩镰孢菌素B(enniatin B);ENB1:恩镰孢菌素B1(enniatin B1);AFG1:黄曲霉毒素G1(aflatoxin G1);AFG2:黄曲霉毒素G2(aflatoxin G2);AFB1:黄曲霉毒素B1(aflatoxin B1);AFB2:黄曲霉毒素B2(aflatoxin B2);ND:未检出;“—”表示未检出或无相关数据;肩标a代表基于阳性样本 (下同)。
小麦籽粒也易受到ZEN污染,ZEN与DON都由几种常见镰刀菌产生,二者往往共同发生,ZEN污染水平也与赤霉病流行相关;小麦中AFs污染水平很低;对于OTA毒素,虽然有限量标准,但国内关于其污染数据有限。FBs产毒真菌在小麦上的发病率较低,在田间缺乏致病性;实验发现,小麦培养物中不会产生大量的FBs;因此,小麦中发生显著的FBs污染可能性较低[12]。伏马毒素B1(fumonisin B1,FB1)比伏马毒素B2(fumonisin B2,FB2)、伏马毒素B3(fumonisin B3,FB3)污染更加严重,有些地区小麦中FBs检出率较高,这可能是由于小麦和玉米轮作,田间存在相关产毒真菌。
当前,一些新兴真菌毒素也逐渐受到关注。小麦及其制品中链格孢霉毒素污染比较严重,通常是多种毒素共存。2021年八省小麦共检出10种新兴毒素,其中TeA检出率高达100%,这与2019年长三角四省链格孢霉毒素(TeA、TEN和ALT)检出率均100%的结果相一致,可能与链格孢菌菌株产毒能力有关。链格孢菌是农业生产中常见的致病菌,严重感染会造成小麦黑点病,影响小麦品质。链格孢霉毒素在小麦加工过程中会保留下来,研究发现小麦粉中毒素含量要明显高于馒头、面包等其他小麦制品[13]。AME和AOH可能会引起细胞突变转化,与人类食管癌病因存在相关性。尽管关于小麦中新兴镰刀菌毒素和链格孢霉毒素的限量法规很少,但其可能会危害人体健康,需要引起重视,建议在田间收获前采用良好农业规范(育种、轮作、耕作、种植时间、杀虫剂、杀菌剂、除草剂等)和加工过程良好生产规范作为控制这些污染物的首选方法。
降雨量和气温是影响田间作物污染水平最主要因素,适宜的温度和高湿度的气候条件一般更有利于产毒真菌侵染和生长,小麦抽穗扬花期如遇持续降雨天气,会增加赤霉病发病率。2018年湖北省是赤霉病最流行区域,气候变化导致降水量增加,小麦田间检测出大量禾谷镰刀菌。有调查显示,安徽、浙江、江苏和上海形成了小麦DON严重污染区,主要由于该区域的特征性降水和气温条件;高暴露聚集区则位于华北地区,可能是南北饮食习惯差异引起的,北方地区的小麦消费量明显高于南方[14]。了解产毒真菌生长的气候条件以及真菌毒素的分布特点,制定合理、有效的措施预防其大范围的污染。
1.1.2 国内小麦粉中真菌毒素污染现状
受污染的小麦加工成小麦粉,毒素并不会被完全去除,即使加工成小麦制品(面条、馒头等),污染仍旧存在。真菌毒素多分布于小麦皮层,去除籽粒表皮能够有效降低小麦粉中毒素含量。国内近几年小麦粉中真菌毒素污染状况如表2所示,DON和ZEN依旧是小麦粉中主要污染物。与精制小麦粉相比,全麦粉中毒素含量可能更高,研究还发现了镰刀菌毒素和链格孢霉菌毒素共存(DON和TeA)。小麦粉是谷物消费模式中真菌毒素暴露的主要膳食来源[14],这是因为大米虽然作为主食,但真菌毒素污染并没有小麦粉中严重,玉米大部分作为饲料用途被消费。持续监测小麦粉中真菌毒素的污染状况,确保饮食暴露风险在安全范围内。
表2 国内小麦粉中真菌毒素污染现状
Table 2 Current status of mycotoxin contamination in domestic wheat flour
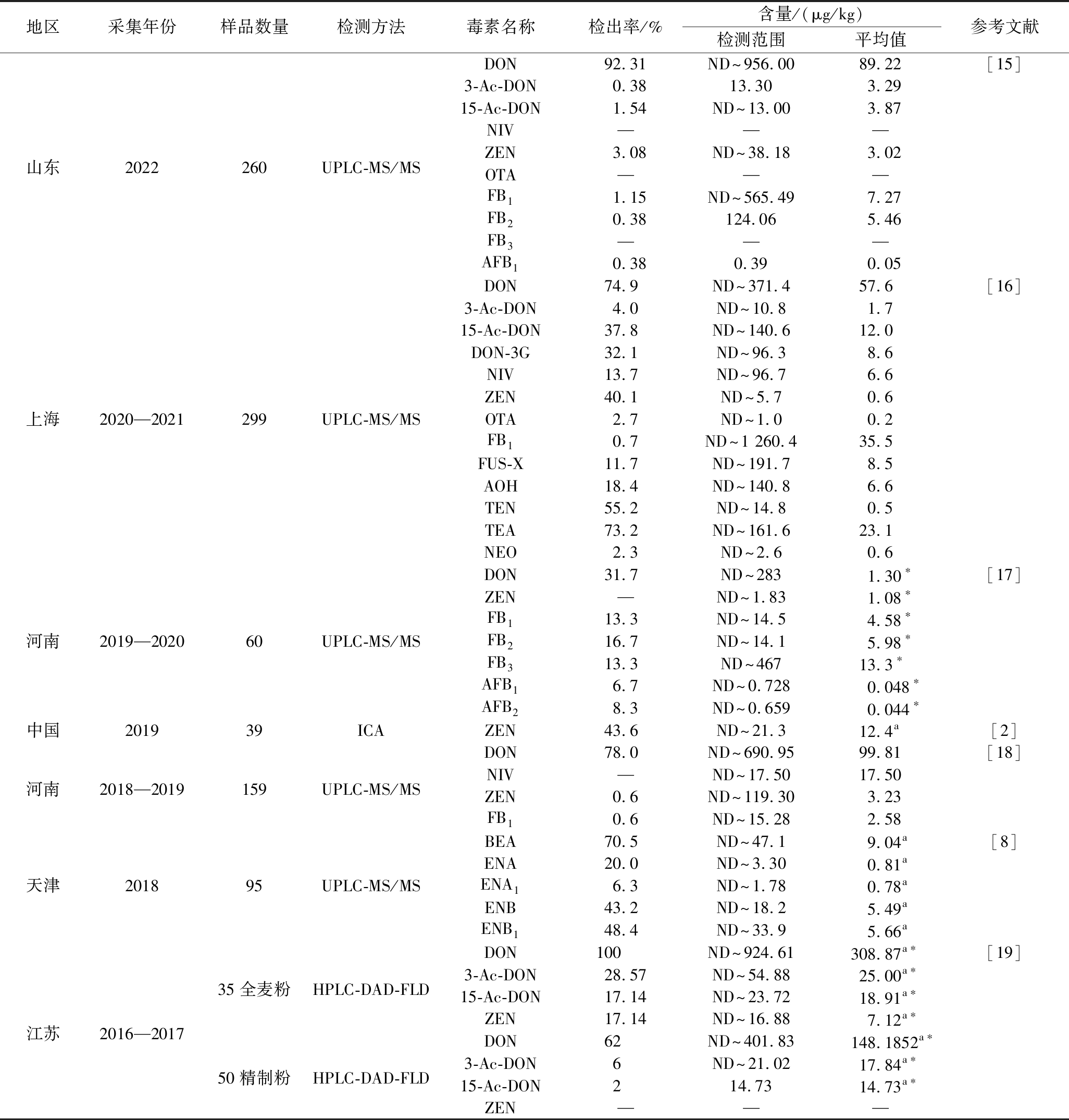
地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献DON92.31ND~956.0089.22[15]3-Ac-DON0.3813.303.2915-Ac-DON1.54ND~13.003.87NIV———山东2022260UPLC-MS/MSZEN3.08ND~38.183.02OTA———FB11.15ND~565.497.27FB20.38124.065.46FB3———AFB10.380.390.05DON74.9ND~371.457.6[16]3-Ac-DON4.0ND~10.81.715-Ac-DON37.8ND~140.612.0DON-3G32.1ND~96.38.6NIV13.7ND~96.76.6ZEN40.1ND~5.70.6上海2020—2021299UPLC-MS/MSOTA2.7ND~1.00.2FB10.7ND~1 260.435.5FUS-X11.7ND~191.78.5AOH18.4ND~140.86.6TEN55.2ND~14.80.5TEA73.2ND~161.623.1NEO2.3ND~2.60.6DON31.7ND~2831.30∗[17]ZEN—ND~1.831.08∗FB113.3ND~14.54.58∗河南2019—202060UPLC-MS/MSFB216.7ND~14.15.98∗FB313.3ND~46713.3∗AFB16.7ND~0.7280.048∗AFB28.3ND~0.6590.044∗中国201939ICAZEN43.6ND~21.312.4a[2]DON78.0ND~690.9599.81[18]河南2018—2019159UPLC-MS/MSNIV—ND~17.5017.50ZEN0.6ND~119.303.23FB10.6ND~15.282.58BEA70.5ND~47.19.04a[8]ENA20.0ND~3.300.81a天津201895UPLC-MS/MSENA16.3ND~1.780.78aENB43.2ND~18.25.49aENB148.4ND~33.95.66aDON100ND~924.61308.87a∗[19]35全麦粉HPLC-DAD-FLD3-Ac-DON28.57ND~54.8825.00a∗15-Ac-DON17.14ND~23.7218.91a∗江苏2016—2017ZEN17.14ND~16.887.12a∗DON62ND~401.83148.1852a∗50精制粉HPLC-DAD-FLD3-Ac-DON6ND~21.0217.84a∗15-Ac-DON214.7314.73a∗ZEN———
注:HPLC-DAD-FLD:高效液相色谱-串联二极管阵列检测器和荧光检测器(high performance liquid chromatography-diode array detector-fluorescence detector);*代表中位数(下同)。
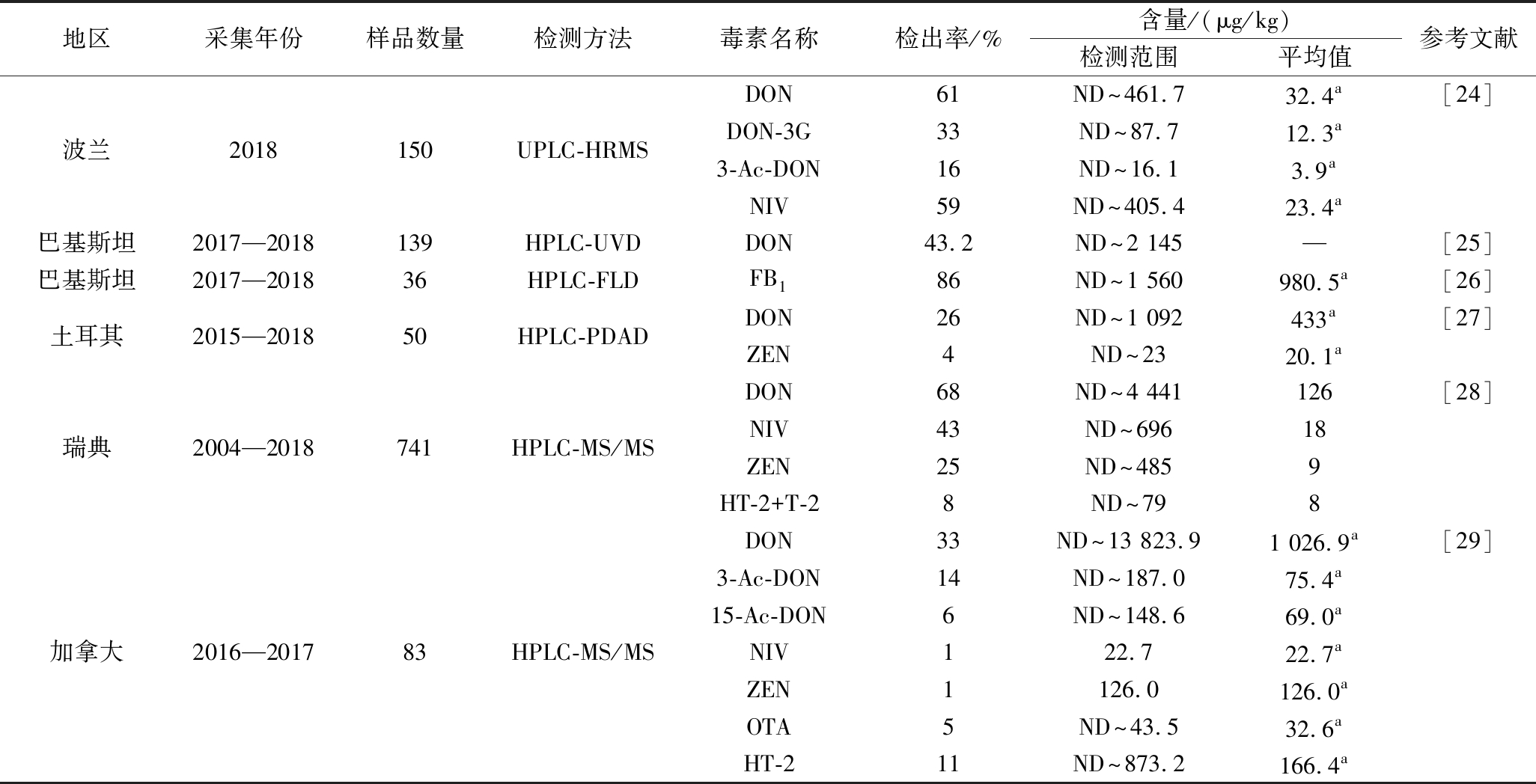
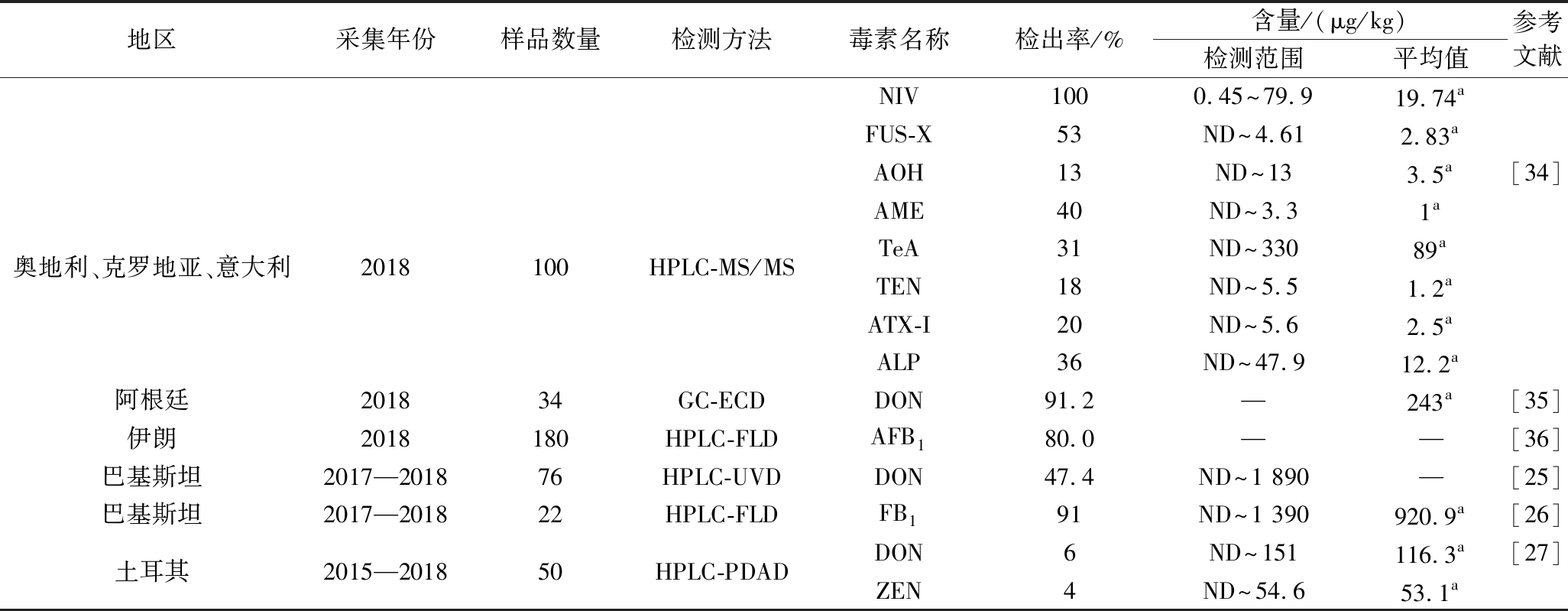
1.2 国外污染现状
小麦中真菌毒素污染是一个全球性问题,国外小麦及小麦粉中真菌毒素污染状况如表3和表4所示,许多国家制定了相关限量标准以及良好农业操作准则。小麦籽粒中如果检出DON或NIV,表明一定受到镰刀菌的侵染。禾谷镰刀菌通常存在温暖和潮湿的地区,如澳大利亚、东欧和北美,而寒冷的气候地区如西欧适合黄色镰刀菌的生长[37]。随着全球气候变化,不同国家地区真菌种群和真菌毒素污染模式正在发生改变,气温升高嗜热真菌向会较温暖地区迁移,嗜冷真菌向较冷区域迁移,气候变化还会削弱小麦作物抗性,使其更容易受到真菌病害影响[38],需要及时制定可行的管控策略,确保小麦质量安全。
表3 国外小麦中真菌毒素污染现状
Table 3 Current status of mycotoxin contamination in wheat abroad

地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献瑞士2020—20217HPLC-MS/MSZEN71.4ND~147.7741.65[20]ENB1003.35~937.85456.79DON70.8ND~15 900794a[21]DON-3G64.1ND~2 120210a3-Ac-DON10.7ND~655175a15-Ac-DON8.99ND~321109aNIV29.8ND~7 5241015aZEN1.12ND~1.501.10aMON2.81ND~10843.9aBEA11.2ND~2.300.80a埃塞俄比亚2020178UPLC-MS/MSENA52.3ND~5.700.40aENA125.8ND~44.73.00aENB43.3ND~3256.50aENB141.0ND~1995.10aENB22.81ND~14.44.00aCUL44.9ND~2 860419aTEN28.1ND~12.62.80aAME10.7ND~2.7011.10aAFB11.12ND~6.303.60aAFB20.560.500.50aDON18ND~375—[22]NIV12ND~120—俄罗斯2018—201974HPLC-MS/MST-24ND~19—HT-236ND~148—MON16ND~39—BEA49ND~49—DON41ND~8 872470a[23]DON-3G7ND~1 072137a3-Ac-DON7ND~10128a15-Ac-DON4ND~21751aNIV1ND~453275aZEN5ND~73864aOTA11ND~456a欧洲2012—2019857LC-MS/MST-27ND~55146aFB127ND~9 122561aFB214ND~59059aFB34ND~41767aAFG11ND~143aAFG23ND~143aAFB12ND~62aAFB23ND~519a
续表3
地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献DON61ND~461.732.4a[24]波兰2018150UPLC-HRMSDON-3G33ND~87.712.3a3-Ac-DON16ND~16.13.9aNIV59ND~405.423.4a巴基斯坦2017—2018139HPLC-UVDDON43.2ND~2 145—[25]巴基斯坦2017—201836HPLC-FLDFB186ND~1 560980.5a[26]土耳其2015—201850HPLC-PDADDON26ND~1 092433a[27]ZEN4ND~2320.1aDON68ND~4 441126[28]瑞典2004—2018741HPLC-MS/MSNIV43ND~69618ZEN25ND~4859HT-2+T-28ND~798DON33ND~13 823.91 026.9a[29]3-Ac-DON14ND~187.075.4a15-Ac-DON6ND~148.669.0a加拿大2016—201783HPLC-MS/MSNIV122.722.7aZEN1126.0126.0aOTA5ND~43.532.6aHT-211ND~873.2166.4a
注:UPLC-HRMS:超高效液相色谱-高分辨质谱(ultra-high performance liquid chromatography high-resolution mass spectrometry);HPLC-UVD:高效液相色谱紫外可见吸收检测器(high performance liquid chromatography-ultraviolet-visible detector);HPLC-FLD:高效液相色谱荧光检测器(high performance liquid chromatography -fluorescence detector);HPLC-PDAD:高效液相色谱二极管阵列检测器(high performance liquid chromatography-photo-diode array detector);ENB2:恩镰孢菌素B2(enniatin B2)。
表4 国外小麦粉中真菌毒素污染现状
Table 4 Current status of mycotoxin contamination in wheat flour abroad
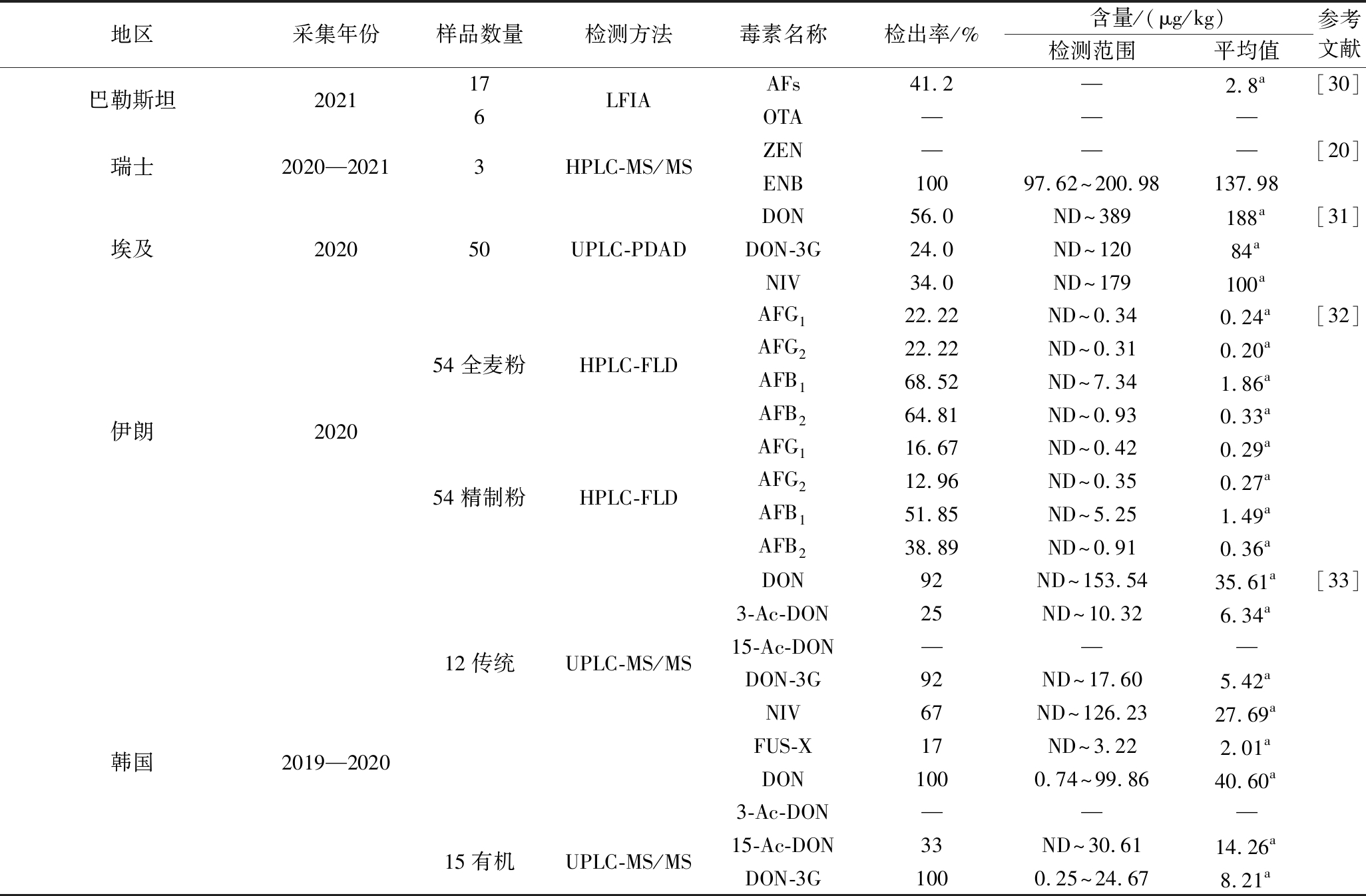
地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献巴勒斯坦202117LFIAAFs41.2—2.8a[30]6OTA———瑞士2020—20213HPLC-MS/MSZEN———[20]ENB10097.62~200.98137.98DON56.0ND~389188a[31]埃及202050UPLC-PDADDON-3G24.0ND~12084aNIV34.0ND~179100aAFG122.22ND~0.340.24a[32]54全麦粉HPLC-FLDAFG222.22ND~0.310.20aAFB168.52ND~7.341.86a伊朗2020AFB264.81ND~0.930.33aAFG116.67ND~0.420.29a54精制粉HPLC-FLDAFG212.96ND~0.350.27aAFB151.85ND~5.251.49aAFB238.89ND~0.910.36aDON92ND~153.5435.61a[33]3-Ac-DON25ND~10.326.34a12传统UPLC-MS/MS15-Ac-DON———DON-3G92ND~17.605.42aNIV67ND~126.2327.69a韩国2019—2020FUS-X17ND~3.222.01aDON1000.74~99.8640.60a3-Ac-DON———15有机UPLC-MS/MS15-Ac-DON33ND~30.6114.26aDON-3G1000.25~24.678.21a
续表4
地区采集年份样品数量检测方法毒素名称检出率/%含量/(μg/kg)检测范围平均值参考文献NIV1000.45~79.919.74aFUS-X53ND~4.612.83aAOH13ND~133.5a[34]AME40ND~3.31a奥地利、克罗地亚、意大利2018100HPLC-MS/MSTeA31ND~33089aTEN18ND~5.51.2aATX-I20ND~5.62.5aALP36ND~47.912.2a阿根廷201834GC-ECDDON91.2—243a[35]伊朗2018180HPLC-FLDAFB180.0——[36]巴基斯坦2017—201876HPLC-UVDDON47.4ND~1 890—[25]巴基斯坦2017—201822HPLC-FLDFB191ND~1 390920.9a[26]土耳其2015—201850HPLC-PDADDON6ND~151116.3a[27]ZEN4ND~54.653.1a
注:LFIA:侧流竞争免疫层析法(lateral flow immunoassay);GC-ECD:气相色谱电子捕获检测器(gas chromatography-electron capture detector);ALP:链格苝醇(alterperylenol)。
2 多种真菌毒素色谱同时检测技术研究
2.1 小麦中多种真菌毒素样品前处理技术
样品前处理直接关系到分析结果的准确性和重现性,合适的样品前处理技术能从基质中提取、净化富集目标毒素,满足分析要求,样品前处理技术主要包含样品采集、提取和净化等内容,各种前处理技术在小麦(粉)中的应用如表5所示。
表5 小麦(粉)中真菌毒素液质联用同时分析技术
Table 5 LC-MS for the simultaneous analysis of mycotoxins in wheat (flour)

真菌毒素检测方法前处理技术方法设定参数LOQ/(μg/kg);回收率/%参考文献DAS、NEO、ENA、ENA1、ENB、ENB1、BEA、T-2、HT-2等19种毒素UPLC-Q-Orbitrap-HRMS超纯水纯化、0.1%(体积分数)甲酸乙腈涡旋2 min、超声10 minQuEChERS萃取试剂盒QuEChERS选择性分散固相萃取试剂盒Zorbax Eclipse Plus C18(100 mm×2.1 mm,1.8 μm)A为2 mmol/L乙酸铵+0.1%(体积分数,下同)乙酸水溶液,B为甲醇梯度洗脱22 minESI+ Full MS/ddMS21.5~6;80.5~98.7[39]DON、NIV、3-Ac-DON、15-Ac-DON、T-2、HT-2、EAs等19种毒素UPLC-Q-Orbitrap-HRMS0.2%甲酸水浸泡、乙腈振荡30 min4 g MgSO4和1 g NaCl冷冻2 h去除脂质Acquity UPLC HSS T3(100 mm×2.1 mm,1.8 μm)A为5 mmol/L甲酸铵+0.2%甲酸水溶液,B为5 mmol/L甲酸铵+0.2%甲酸甲醇梯度洗脱12 minESI+ PRMC为5 mmol/L甲酸铵水溶液,D为甲醇ESI- PRM0.5~50.0;72~103[40]DON、3-Ac-DON、NIV、FUS-X、ZEN、ZAN、T-2、HT-2、DAS、NEOHPLC-QqQ-MS/MS乙腈-水(84∶16)搅拌1 h无净化Gemini C18(150 mm×4.60 mm,3 μm)A为5 mmol/L乙酸铵-甲醇(90∶10)溶液,B为甲醇-水(90∶10)溶液梯度洗脱18 minAPCI(+ -) SRM0.5~5;59.1~113[41]T-2、NEO、HT-2、DASUPLC-QqQ-MS/MS乙腈-水(84∶16)摇床混合30 minMycoSep 227 SPE小柱Acquity UPLC BEH C18柱(100 mm×2.1 mm,1.7 μm)A为5 mmol/L甲酸铵+0.1%甲酸水溶液,B为0.1%甲酸的甲醇梯度洗脱17 minESI+ MRM模式—;97~103[42]AFB1、AFB2、AFG1、AFG2、ZEN、OTA、FB1、FB2、DON、T-2、HT-2UPLC-QqQ-MS/MS乙腈-水-乙酸(79.5∶20∶0.5)摇床摇动60 minMyco6in1+IACKinetex XB-C18(100 mm× 2.1 mm,2.6 μm)流动相A为10 mmol/L甲酸铵+0.1%甲酸水溶液,B为10 mmol/L甲酸铵+0.1%甲酸甲醇,ESI+MRM模式2~100;72~123[43]AFB1、AFB2、AFG1、AFG2、DON、FB1、FB2、ZEN、T-2、HT-2、OTAUPLC-QqQ-MS/MS乙腈-水-甲酸(79∶20∶1)机械振荡90 min乙腈-水-甲酸(20∶79∶1)等分稀释提取物Acquity UPLC HSS T3(100 mm×2.1 mm,1.8 μm)A为5 mmol/L甲酸铵水溶液,B为甲醇梯度洗脱18 minESI(+ -)MRM模式0.5~200;93.5~111.1[44]AFB1、AFB2、AFG1、AFG2、OTA、T-2、HT-2、DON、NIV、PAT等18种毒素UPLC-Q-TOF/MS10%甲酸水涡旋、乙腈振荡25 minQuEChERS萃取盐包Oasis Prime HLB小柱Waters HSS T3 C18(100 mm×2.1 mm,1.8 μm)A为5 mmol/L乙酸铵+1%乙酸水溶液,B为甲醇梯度洗脱22 minESI(+ -)MSE—[45]AFB1、AFB2、FB1、FB2、FB3、DON、NEO、DAS、FUS-X等18种毒素UPLC-Q-TRAP-MS/MS50%(体积分数)乙腈水摇床振摇1 h、加入乙酸MycoSpinTM 400净化柱Gemini HPLC C18(150 mm×4.6 mm,5 μm)A为2 mmol/L乙酸铵+0.5%乙酸水溶液,B为2 mmol/L乙酸铵+0.5%乙酸甲醇梯度洗脱20 minESI(+ -)MRM模式0.5~30;68.9~109.22[46]
注:所有试剂配比均为体积比;Full MS/ddMS2:全扫描/数据依赖性二级扫描;PRM:平行反应监测(parallel reaction monitoring);SRM:选择反应监测(selected reaction monitoring);MSE:全信息串联质谱;“+”表示正离子扫描,“-”表示负离子扫描;LOQ:定量限(limit of quantitation);“—”表示文献中未明确说明。
2.1.1 取样方法
真菌毒素通常在样品中分布不均匀,收集具有代表性的样品与检测结果准确性直接相关。谷物取样步骤是影响变异性的关键,面粉取样只是次要;其次是样品制备过程[47]。小麦田间取样与收割方式有关,通常田地边缘或低洼区域DON水平较高;仓房取样要求位置合理、布点科学,由于谷物和杂质的自动分级作用,较小、较密的物质集中在中心,通常毒素含量也较高。样品制备时,需要先进行清杂处理,赤霉病小麦干瘪、质量较轻,清杂时需格外注意,常采用五点取样法取出代表性样品,再经粉碎过筛、晃匀抽取可供分析的实验室样品(通常1 kg)。研究表明,在真菌毒素分析过程中,实验室样品取样量、制备方法以及标准抽样方法没有被重视[48]。
2.1.2 固液萃取
固液萃取常用于不同样品中提取真菌毒素,萃取的关键是选择对目标毒素具有较强选择性的溶剂。提取溶剂通常是由有机相(乙腈/甲醇)-水-少量酸液(甲酸/乙酸/柠檬酸)组成的混合体系,水可以加速有机相进入小麦基质,少量酸有助于对酸敏感的毒素(如OTA、FBs、TeA等)提取和降低基质效应。常见提取方式主要有摇床振荡、磁力搅拌、超声提取、微波提取和涡旋振荡。实验发现超声提取的毒素回收率高于摇床振荡和涡旋,但超声可能将基质中蛋白质、色素等杂质一同提取出来,影响样品净化[49]。新型分散处理设备Ultra-Turrax也被用来辅助提取样品中真菌毒素,且处理后无需净化操作,因为提取物颜色为透明[50]。
2.1.3 固相萃取
固相萃取(solid phase extraction,SPE)通过不同键合相的吸附剂对提取溶液中分析物进行分离、纯化和富集,是一种广泛使用的前处理方法。SPE具有溶剂消耗少、浓缩效率高、回收率好等优点,常用的固相吸附材料包括反相吸附剂,如C18、C8等,适用于非极性或弱极性毒素萃取;正向吸附剂,如氨基、氰基、氧化铝等,适用于极性毒素萃取;离子交换吸附剂包括丙磺酸、三甲基胺丙基等;其他吸附剂包括Oasis HLB、PRIME、多功能净化柱(MycoSpinTM 400、MycoSep 227等)、免疫亲和柱(immnuo affinity chromatography columns,IACs)等[51]。随着不断发展,新型吸附剂如分子印迹聚合物、适配体、纳米颗粒、活性炭、磁性吸附剂等是当前的研究热点,基于SPE的新技术(固相微萃取、微固相萃取、分散微固相萃取)也逐渐被应用到样品前处理中。净化时,通常需要对这些吸附剂的种类、用量进行优化。
2.1.4 QuEChERS
QuEChERS是一种常用的前处理技术,盐析剂促进液液分配,净化剂与基质中杂质相互作用,达到提取、净化的效果,逐渐被改良应用在真菌毒素分析中。常用的盐析剂为无水硫酸镁和氯化钠,质量比为4∶1;常用的净化剂有C18、N-丙基乙二胺(primary secondary amine,PSA)和石墨化炭黑(graphitized carbon black,GCB)等。PSA可以去除小麦提取物中糖、脂肪酸以及其他形成氢键的成分等,C18用于去除脂质和甾醇,常使用PSA、C18加硫酸镁组合净化基质溶液,GCB因能与平面结构的真菌毒素(ZEN、AOH、AME等)产生吸附作用而在使用中受到限制。
2.1.5 稀释和注射
需处理大量样品时,有时为了减少样品处理步骤,无需净化直接稀释样品进行检测,稀释通常是为了减少基质效应带来的影响[52],同时也避免净化期间某些毒素的高损失。稀释和注射被认为是最简单、最直接、最快速的净化方法,相比其他净化技术更加环保,但是该技术的重现性、准确度和精密度可能存在问题[53]。
2.2 液相色谱检测技术
HPLC定量分析真菌毒素是一种应用成熟的检测技术。正向和反相色谱均有应用,反相HPLC经济实惠、便于操作且溶剂毒性较小,大多数毒素可溶于极性流动相,再通过非极性色谱柱分离。HPLC检测时通常与FLD、UVD、PDAD、DAD等检测器联合使用。FLD和UVD要求毒素结构中存在发色团(OTA、AFB1、AFB2、AFG1、AFG2等有天然荧光特性)才能被用于定量。常在柱前或柱后进行衍生化操作,改善毒素荧光特性,提升检测灵敏度。黎睿等[54]建立了一种HPLC-可变波长检测器串联光化学衍生器串联荧光检测器方法对小麦中8种真菌毒素AFB1、AFB2、AFG1、AFG2、OTA、ZEN、DON和T-2毒素进行同时测定,系统运行28 min,加标回收率为83.2%~102.8%,精密度为2.6%~10.2%,满足粮食中多种真菌毒素快速检测的需求。UPLC联合检测器也逐渐被应用,更高的压力模块和更小尺寸、更小粒径的色谱柱,可缩短样品运行时间,提高色谱分离效率、达到更高灵敏度[55]。GAB-ALLAH等[31]使用UPLC-PDAD检测小麦粉中DON、DON-3G和NIV,218 nm波长等度洗脱(乙腈-水,10∶90)8 min充分分离目标毒素,具有良好的选择性,方法回收率为86.3%~106.0%,相对标准偏差≤4%,满足欧盟标准。
2.3 液质联用检测技术
LC-MS是现代最广泛使用的痕量真菌毒素分析方法,表5列举了小麦(粉)中真菌毒素液质联用同时分析技术。质谱以其依据特征碎片离子确定目标毒素的独特优势,成为多残留物分析的首选技术,还可对毒素代谢物进行研究。质谱常用电离模式为电喷雾电离(electospray ionization,ESI)和大气压化学电离(atmospheric pressure chemical ionization,APCI)。ESI被应用在大多数检测中,电离能量相对较小,可获得完整的分子信息,但会产生基质效应,影响定量结果准确度。APCI对于A型和B型TCTs表现出更好的电离结果[56]。多种真菌毒素化学性质不同,根据离子信号强度和特征子离子碎裂模式选择正离子或负离子检测模式,有时还会在正负间切换进行扫描。
常见的质量分析器串联形式有三重四极杆(triple quadrupole,QqQ)、四极杆-飞行时间(quadrupole-time-of-flight,Q-TOF)、四极杆-静电场轨道肼(quadrupole-orbitrap,Q-Orbitrap)、三重四极杆复合线性离子阱(triple quadrupole linear ion trap,Q-TRAP),检测可实现更加精准目标物质、未知化合物的鉴定和更高的灵敏度。基于TOF和Orbitrap的HRMS全扫描模式下能记录设定质量范围内的全部质谱信号,支持数据回溯分析。目前,QqQ已成为食品中真菌毒素定量分析的常用技术,在多反应监测模式(multiple reaction monitoring,MRM)下有明确的选择性(监测母离子和子离子);进行多毒素分析时,要注意限制单次扫描记录的监测离子对数量,确保每个色谱峰有足够的点数(最少15个),能实现重复分析[57]。
在开发方法时,根据目标毒素的物理化学性质,选择合适的色谱柱、流动相和洗脱梯度,优化质谱扫描方法,达到良好的定性、定量分析。合适的色谱柱是决定分离效果的前提条件。流动相(缓冲溶液)组成和比例不仅影响分析物的色谱图和峰参数,还影响离子源电离效率。与乙腈/水相比,甲醇/水作为流动相能更好地分离真菌毒素。DE SANTIS等[58]研究了不同缓冲液浓度对真菌毒素(AFB1、DON、ZEN、FB1、T-2、HT-2和OTA)峰强度的影响,发现在流动相中加入甲酸和甲酸铵能促进毒素形成质子化前体离子或铵加合物,加入0.3%(体积分数,下同)甲酸和5 mmol/L甲酸铵可获得最高峰强度。优化质谱条件时,将真菌毒素标准溶液注入质谱,选择正负扫描模式、优化碰撞电压和离子源条件。需要注意当质谱条件仅设置为高分辨率和全扫描模式下,可能会增加某些真菌毒素的假阳性概率。
2.4 气相色谱及质谱检测技术
GC、GC-MS/MS用于分析能挥发且不会发生热分解的目标化合物,火焰离子化检测器、ECD和质谱主要用于真菌毒素GC检测[59]。通过GC分析的第一个真菌毒素是ZEN,TCTs是气相色谱广泛应用的一类真菌毒素,通常会硅烷化或酰化衍生生成挥发性更强、热稳定性更好的衍生物[60]。STANCIU等[61]以GC-QqQ-MS/MS同时检测小麦中DON、3-Ac-DON、15-Ac-DON、FUS-X、DAS、NIV、NEO、HT-2、T-2和ZEN,经硅烷化试剂衍生化反应,在质谱SRM模式下定量分析,LOQ为1~20 μg/kg,10种毒素的回收率为69%~127%。GC、GC-MS/MS可一次性同时分析多种真菌毒素,适用于食品、谷物和饲料中TCTs和ZEN的检测分析。
2.5 色谱同时检测技术对比
基于色谱真菌毒素同时检测技术是实验室常用的分析方法,其灵敏度高、准确性好,但也存在设备昂贵、样品前处理复杂费时、无法现场实时检测等局限,表6对比分析了各种色谱同时检测技术,并对其适用范围进行总结。UPLC凭借粒径更小的色谱柱可大幅缩短分析时间、提高分离效率,广泛与质谱联用。由于毒素的高极性和有限的挥发性,GC和GC-MS/MS较少用于真菌毒素检测[63]。LC-MS在痕量检测方面优于HPLC,一次进样可同时分析多种真菌毒素,还可对代谢物和降解产物进行结构分析,QqQ质谱仪和ESI离子源已成为检测真菌毒素的主力军。
表6 色谱同时检测技术对比分析
Table 6 Comparative analysis of chromatographic simultaneous detection techniques
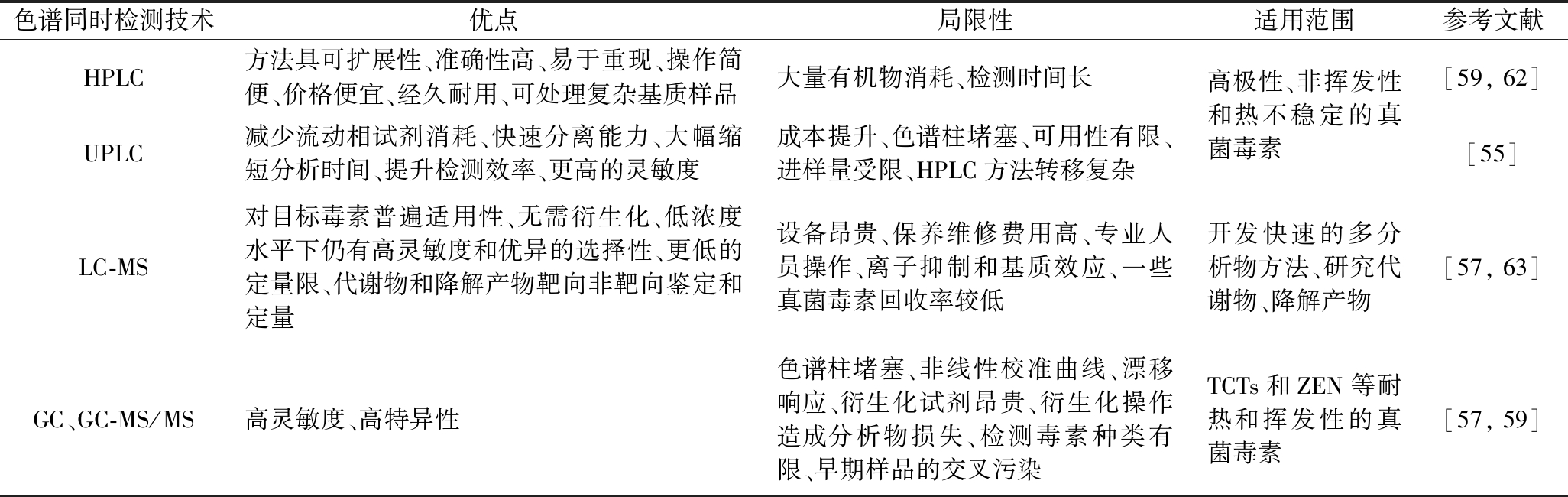
色谱同时检测技术优点局限性适用范围参考文献HPLCUPLC方法具可扩展性、准确性高、易于重现、操作简便、价格便宜、经久耐用、可处理复杂基质样品减少流动相试剂消耗、快速分离能力、大幅缩短分析时间、提升检测效率、更高的灵敏度大量有机物消耗、检测时间长成本提升、色谱柱堵塞、可用性有限、进样量受限、HPLC方法转移复杂高极性、非挥发性和热不稳定的真菌毒素[59, 62][55]LC-MS对目标毒素普遍适用性、无需衍生化、低浓度水平下仍有高灵敏度和优异的选择性、更低的定量限、代谢物和降解产物靶向非靶向鉴定和定量设备昂贵、保养维修费用高、专业人员操作、离子抑制和基质效应、一些真菌毒素回收率较低开发快速的多分析物方法、研究代谢物、降解产物[57, 63]GC、GC-MS/MS高灵敏度、高特异性色谱柱堵塞、非线性校准曲线、漂移响应、衍生化试剂昂贵、衍生化操作造成分析物损失、检测毒素种类有限、早期样品的交叉污染TCTs和ZEN等耐热和挥发性的真菌毒素[57, 59]
3 展望
小麦中天然存在多种真菌毒素,真菌毒素的产生不仅造成小麦质量损失,而且严重危害人体健康。本文搜集国内外近几年小麦、小麦粉中真菌毒素污染数据进行分析,探究小麦中主要真菌毒素污染规律,研究结果对于制定农业规范、保障小麦质量安全具有参考价值。此外,本文总结了小麦中真菌毒素色谱同时检测方法,其中液质联用是实验室中常用分析方法。未来研究建议如下:a)关于小麦真菌毒素的限量。目前仅包含一些常见真菌毒素,其他已知毒素和新兴毒素不在立法范围内;仅规定单个毒素的限量,应考虑多种毒素共存时的累计毒性效应;分类对象不够细化,原粮、成品粮未区分,特殊人群(如婴幼儿)应加以区分,建议定期评估真菌毒素暴露风险,制定严格立法,确保标准执行;b)基于色谱分析时样品前处理。基质干扰,前处理复杂、费时,一些毒素回收率低等问题,有待开发快速、准确、便捷的净化、富集技术(开发新型材料、结合多种前处理技术、提高自动化程度),提升检测效率;c)继续开发隐蔽性、新兴真菌毒素的检测技术,保证低含量毒素的准确检测,健全检测机制,加强新兴毒素监管,建立从田间到加工全流程的风险控制体系,探索可能存在的污染规律,为粮食和食品安全提供可靠的技术支持。
[1] JI X F, JIN C H, XIAO Y P, et al.Natural occurrence of regulated and emerging mycotoxins in wheat grains and assessment of the risks from dietary mycotoxins exposure in China[J].Toxins, 2023, 15(6):389.
[2] HONG X, MAO Y H, YANG C Q, et al.Contamination of Zearalenone from China in 2019 by a visual and digitized immunochromatographic assay[J].Toxins, 2020, 12(8):521.
[3] 赵柬云, 韩小敏, 徐文静, 等.2019年中国四省小麦中12种真菌毒素污染情况调查[J].中国食品卫生杂志, 2021, 33(6):765-770.
ZHAO J Y, HAN X M, XU W J, et al.Investigation of 12 mycotoxins in wheat grains from four provinces of China in 2019[J].Chinese Journal of Food Hygiene, 2021, 33(6):765-770.
[4] 唐占敏. 长三角地区谷物中典型真菌毒素识别及污染研究[D].上海:上海海洋大学, 2020.
TANG Z M.Identification and pollution of typical mycotoxins in cereals in Yangtze River Delta region[D].Shanghai:Shanghai Ocean University, 2020.
[5] 任贝贝, 王丽英, 路杨, 等.河北省小麦、玉米及其制品中16种真菌毒素污染水平调查与分析[J].食品安全质量检测学报, 2021, 12(5):1669-1676.
REN B B, WANG L Y, LU Y, et al.Investigation and analysis of 16 kinds of mycotoxins pollution levels in wheat, corn and products in Hebei province[J].Journal of Food Safety &Quality, 2021, 12(5):1669-1676.
[6] ZHAO J Y, CHENG T X, XU W J, et al.Natural co-occurrence of multi-mycotoxins in unprocessed wheat grains from China[J].Food Control, 2021, 130:108321.
[7] 程天笑, 韩小敏, 王硕, 等.2018年中国4省脱粒小麦中9种真菌毒素污染情况调查[J].食品安全质量检测学报, 2020, 11(12):3992-3999.
CHENG T X, HAN X M, WANG S, et al.Investigation on contamination situation of 9 mycotoxins in wheat kernel from 4 provinces of China in 2018[J].Journal of Food Safety &Quality, 2020, 11(12):3992-3999.
[8] LIN X H, ZHANG Q, ZHANG Y, et al.Further data on the levels of emerging Fusarium mycotoxins in cereals collected from Tianjin, China[J].Food Additives &Contaminants:Part B, Surveillance, 2021, 14(1):74-80.
[9] 王瑞虎, 李萌萌, 关二旗, 等.乙酰化脱氧雪腐镰刀菌烯醇污染、毒性及转化研究进展[J].食品科学, 2023, 44(1):345-352.
WANG R H, LI M M, GUAN E Q, et al.Progress in research on contamination, toxicity and transformation of acetylated deoxynivalenol[J].Food Science, 2023, 44(1):345-352.
[10] OVANDO-MART NEZ M, OZSISLI B, ANDERSON J, et al.Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum[J].Toxins, 2013, 5(12):2522-2532.
NEZ M, OZSISLI B, ANDERSON J, et al.Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum[J].Toxins, 2013, 5(12):2522-2532.
[11] WANG R H, LI M M, JIN R, et al.Interactions among the composition changes in fungal communities and the main mycotoxins in simulated stored wheat grains[J].Journal of the Science of Food and Agriculture, 2024, 104(1):373-382.
[12] SHEPHARD G S, VAN DER WESTHUIZEN L, GATYENI P M, et al.Do fumonisin mycotoxins occur in wheat?[J].Journal of Agricultural and Food Chemistry, 2005, 53(23):9293-9296.
[13] 张林炜. 市售小麦制品中链格孢霉毒素残留和膳食暴露评估[D].合肥:安徽农业大学, 2023.
ZHANG L W.Assessment of Alternaria toxin residues and dietary exposure in wheat products on the market[D].Hefei:Anhui Agricultural University, 2023.
[14] WANG X D, YANG D J, QIN M, et al.Risk assessment and spatial analysis of deoxynivalenol exposure in Chinese population[J].Mycotoxin Research, 2020, 36(4):419-427.
[15] 杨晓倩, 刘岚铮, 曹小丽, 等.2022年山东省市售小麦粉真菌毒素污染情况调查[J].预防医学论坛, 2023, 29(8):577-581.
YANG X Q, LIU L Z, CAO X L, et al.Investigation on mycotoxin pollution of wheat flour on market in Shandong province in 2022[J].Preventive Medicine Tribune, 2023, 29(8):577-581.
[16] ZHOU H Y, XU A Q, LIU M C, et al.Mycotoxins in wheat flours marketed in Shanghai, China:Occurrence and dietary risk assessment[J].Toxins, 2022, 14(11):748.
[17] 张欣烨, 彭靖, 张洁, 等.2019—2020年河南省谷类食品中7种真菌毒素污染状况调查[J].中国卫生检验杂志, 2021, 31(18):2274-2276.
ZHANG X Y, PENG J, ZHANG J, et al.Investigation into contamination status of 7 mycotoxins in cereals in Henan Province from 2019 to 2020[J].Chinese Journal of Health Laboratory Technology, 2021, 31(18):2274-2276.
[18] 李俊玲, 王书舟, 吴俊威, 等.河南省粮食及其制品中真菌毒素污染情况调查[J].中国食品卫生杂志, 2020, 32(4):418-421.
LI J L, WANG S Z, WU J W, et al.Investigation of mycotoxins in grain and its products in Henan Province[J].Chinese Journal of Food Hygiene, 2020, 32(4):418-421.
[19] ZHANG Y Y, PEI F, FANG Y, et al.Comparison of concentration and health risks of 9 Fusarium mycotoxins in commercial whole wheat flour and refined wheat flour by multi-IAC-HPLC[J].Food Chemistry, 2019, 275:763-769.
[20] ANDRÉ A, MÜLLER N, CHETSCHIK I.Occurrence of Zearalenone and enniatin B in Swiss wheat grains and wheat flours[J].Applied Sciences, 2022, 12(20):10566.
[21] GETAHUN M, FININSA C, MOHAMMED A, et al.Fungal species and multi-mycotoxin in bread wheat (Triticum aestivum L.) in Ethiopia[J].World Mycotoxin Journal, 16(2):179-194.
[22] GAVRILOVA O P, GAGKAEVA T Y, ORINA A S, et al.Diversity of Fusarium species and their mycotoxins in cereal crops from the Asian territory of Russia[J].Doklady Biological Sciences, 2023, 508(1):9-19.
[23] KOLETSI P, SCHRAMA J W, GRAAT E A M, et al.The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of deoxynivalenol (DON) on the health and growth of farmed fish species-a review[J].Toxins, 2021, 13(6):403.
[24] BRY A M, KSIENIEWICZ-WOÖNIAK E, YOSHINARI T, et al.Contamination of wheat cultivated in various regions of Poland during 2017 and 2018 agricultural seasons with selected trichothecenes and their modified forms[J].Toxins, 2019, 11(2):88.
A M, KSIENIEWICZ-WOÖNIAK E, YOSHINARI T, et al.Contamination of wheat cultivated in various regions of Poland during 2017 and 2018 agricultural seasons with selected trichothecenes and their modified forms[J].Toxins, 2019, 11(2):88.
[25] IQBAL S Z, USMAN S, RAZIS A F A, et al.Assessment of deoxynivalenol in wheat, corn and its products and estimation of dietary intake[J].International Journal of Environmental Research and Public Health, 2020, 17(15):5602.
[26] IQBAL S Z, REHMAN B, SELAMAT J, et al.Assessment of fumonisin B1 concentrations in wheat and barley products in the punjab region of Pakistan[J].Journal of Food Protection, 2020, 83(8):1284-1288.
[27] GOLGE O, KABAK B.Occurrence of deoxynivalenol and Zearalenone in cereals and cereal products from Turkey[J].Food Control, 2020, 110:106982.
[28] KARLSSON I, MELLQVIST E, PERSSON P.Temporal and spatial dynamics of Fusarium spp.and mycotoxins in Swedish cereals during 16 years[J].Mycotoxin Research, 2023, 39(1):3-18.
[29] SHI H T, SCHWAB W, YU P Q.Natural occurrence and co-contamination of twelve mycotoxins in industry-submitted cool-season cereal grains grown under a low heat unit climate condition[J].Toxins, 2019, 11(3):160.
[30] SALMAN M K, MUDALAL S.Quality control and mycotoxin levels in food in the Palestinian market[J].Food Additives &Contaminants: Part B, 2022,15(2):123-128.
[31] GAB-ALLAH M A, TAHOUN I F, YAMANI R N, et al.Natural occurrence of deoxynivalenol, nivalenol and deoxynivalenol-3-glucoside in cereal-derived products from Egypt[J].Food Control, 2022, 137:108974.
[32] HESHMATI A, MOZAFFARI NEJAD A S, MEHRI F.Occurrence, dietary exposure, and risk assessment of aflatoxins in wheat flour from Iran[J].International Journal of Environmental Analytical Chemistry, 2023, 103(20):9395-9408.
[33] GAB-ALLAH M A, MEKETE K G, CHOI K, et al.Occurrence of major type-B trichothecenes and deoxynivalenol-3-glucoside in cereal-based products from Korea[J].Journal of Food Composition and Analysis, 2021, 99:103851.
[34] PUNTSCHER H, COBANKOVIC I, MARKO D, et al.Quantitation of free and modified Alternaria mycotoxins in European food products by LC-MS/MS[J].Food Control, 2019, 102:157-165.
[35] CIRIO M, VILLARREAL M, SEAL T M L, et al.Incidence of deoxynivalenol in wheat flour in Argentina and GC-ECD method validation[J].Journal of AOAC International, 2019, 102(6):1721-1724.
[36] JAHANBAKHSH M, AFSHAR A, MOMENI FEELI S, et al.Probabilistic health risk assessment (Monte Carlo simulation method) and prevalence of aflatoxin B1 in wheat flours of Iran[J].International Journal of Environmental Analytical Chemistry, 2021, 101(8):1074-1085.
[37] BRY A M,
A M, ![]() A, et al.Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in Polish winter wheat[J].Toxins, 2018, 10(2):81.
A, et al.Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in Polish winter wheat[J].Toxins, 2018, 10(2):81.
[38] CASU A, LEGGIERI M C, TOSCANO P, et al.Changing climate, shifting mycotoxins:A comprehensive review of climate change impact on mycotoxin contamination[J].Comprehensive Reviews in Food Science and Food Safety, 2024, 23(2):e13323.
[39] 崔东伟. 谷物及其制品中真菌毒素的检测技术研究[D].沈阳:中国医科大学, 2021.
CUI D W.Research on precise detection technology of mycotoxins in grains and their products[D].Shenyang:China Medical University, 2021.
[40] TSAGKARIS A S, PRUSOVA N, DZUMAN Z, et al.Regulated and non-regulated mycotoxin detection in cereal matrices using an ultra-high-performance liquid chromatography high-resolution mass spectrometry (UHPLC-HRMS) method[J].Toxins, 2021, 13(11):783.
[41] SANTINI A, FERRACANE R, SOMMA M C, et al.Multitoxin extraction and detection of trichothecenes in cereals:An improved LC-MS/MS approach[J].Journal of the Science of Food and Agriculture, 2009, 89(7):1145-1153.
[42] GAB-ALLAH M A, CHOI K, KIM B.Development of isotope dilution-liquid chromatography/tandem mass spectrometry for the accurate determination of type-a trichothecenes in grains[J].Food Chemistry, 2021, 344:128698.
[43] HEIDARI G, HASHEMI HAZAVEH S J, DARAEI B, et al.Validation of an UHPLC-MS/MS method for simultaneous analysis of 11 mycotoxins in wheat flour using immunoaffinity column[J].Iranian Journal of Pharmaceutical Research, 2019, 18(Suppl1):182-189.
[44] ![]() ,
, ![]() J, et al.Mycotoxins, pesticide residues, and heavy metals analysis of Croatian cereals[J].Microorganisms, 2021, 9(2):216.
J, et al.Mycotoxins, pesticide residues, and heavy metals analysis of Croatian cereals[J].Microorganisms, 2021, 9(2):216.
[45] ZHANG L X, HUANG Z H, LUO S Q, et al.Establishment of non-targeted screening database and confirmation method for 18 mycotoxins in grains using ultra performance liquid chromatography-quadrupole-time of flight mass spectrometry[J].Se Pu, 2023, 41(1):66-75.
[46] 张大伟, 高和杨, 周旌, 等.超高效液相色谱-串联质谱法同时检测饲料原料、饲料成品中18种真菌毒素含量[J].食品安全质量检测学报, 2018, 9(22):5867-5876.
ZHANG D W, GAO H Y, ZHOU J, et al.Simultaneous determination of 18 kinds of mycotoxins in feed ingredients and feed products by ultra performance liquid chromatography-tandem mass spectrometry[J].Journal of Food Safety &Quality, 2018, 9(22):5867-5876.
[47] HALLIER A, CELETTE F, DAVID C.Effects of sampling and extraction on deoxynivalenol quantification[J].Food Chemistry, 2011, 127(1):303-307.
[48] KUMPHANDA J, MATUMBA L, MONJEREZI M, et al.Are sample size and sample preparation for mycotoxin quantitation in grain products getting trivialized?[J].Food Control, 2021, 130:108400.
[49] 王蓓蓓. 复合免疫亲和-液相色谱-串联质谱法测定黑米中真菌毒素的研究[D].汉中:陕西理工大学, 2022.
WANG B B.Determination of mycotoxins in black rice by compound immunoaffinity-liquid chromatography-tandem mass spectrometry[D].Hanzhong:Shaanxi University of Technology, 2022.
[50] SERRANO A B, FONT G, MA ES J, et al.Comparative assessment of three extraction procedures for determination of emerging Fusarium mycotoxins in Pasta by LC-MS/MS[J].Food Control, 2013, 32(1):105-114.
ES J, et al.Comparative assessment of three extraction procedures for determination of emerging Fusarium mycotoxins in Pasta by LC-MS/MS[J].Food Control, 2013, 32(1):105-114.
[51] BIAN Y, ZHANG Y, ZHOU Y, et al.Recent insights into sample pretreatment methods for mycotoxins in different food matrices:A critical review on novel materials[J].Toxins, 2023, 15(3):215.
[52] SULYOK M, BERTHILLER F, KRSKA R, et al.Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize[J].Rapid Communications in Mass Spectrometry, 2006, 20(18):2649-2659.
[53] GREER B, CHEVALLIER O, QUINN B, et al.Redefining dilute and shoot:The evolution of the technique and its application in the analysis of foods and biological matrices by liquid chromatography mass spectrometry[J].TrAC Trends in Analytical Chemistry, 2021, 141:116284.
[54] 黎睿, 谢刚, 王松雪.高效液相色谱法同时检测粮食中常见8种真菌毒素的含量[J].食品科学, 2015, 36(6):206-210.
LI R, XIE G, WANG S X.Simultaneous analysis of 8 mycotoxins in grains by high performance liquid chromatography[J].Food Science, 2015, 36(6):206-210.
[55] SANTOS PEREIRA C, C CUNHA S, FERNANDES J O.Prevalent mycotoxins in animal feed:Occurrence and analytical methods[J].Toxins, 2019, 11(5):290.
[56] BERTHILLER F, SCHUHMACHER R, BUTTINGER G, et al.Rapid simultaneous determination of major type A- and B-trichothecenes as well as Zearalenone in maize by high performance liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography. A, 2005, 1062(2):209-216.
[57] VARGAS MEDINA D A, BASSOLLI BORSATTO J V, MACIEL E V S, et al.Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food[J].TrAC Trends in Analytical Chemistry, 2021, 135:116156.
[58] DE SANTIS B, DEBEGNACH F, GREGORI E, et al.Development of a LC-MS/MS method for the multi-mycotoxin determination in composite cereal-based samples[J].Toxins, 2017, 9(5):169.
[59] SINGH J, MEHTA A.Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods:A review[J].Food Science &Nutrition, 2020, 8(5):2183-2204.
[60] SCOTT P M.Gas Chromatography of Mycotoxins [M].Amsterdam:Elsevier.1993:373-425.
[61] STANCIU O, JUAN C, BERRADA H, et al.Study on trichothecene and Zearalenone presence in Romanian wheat relative to weather conditions[J].Toxins, 2019, 11(3):163.
[62] YANG Y, LI G L, WU D, et al.Recent advances on toxicity and determination methods of mycotoxins in foodstuffs[J].Trends in Food Science &Technology, 2020, 96:233-252.
[63] DIB A A, ASSAF J C, DEBS E, et al.A comparative review on methods of detection and quantification of mycotoxins in solid food and feed:A focus on cereals and nuts[J].Mycotoxin Research, 2023, 39(4):319-345.