黄曲霉毒素(aflatoxins,AFs)是由黄曲霉、寄生曲霉等产毒曲霉产生的结构类似的次级代谢产物[1-3]。其中,黄曲霉毒素M1(aflatoxin M1,AFM1)是哺乳动物摄入被黄曲霉毒素B1(aflatoxin B1,AFB1)污染的饲料后,在其肝脏被转化而来的羟基化代谢产物,并通过哺乳动物的乳汁排出体内,具有致癌性、免疫毒性、遗传毒性和致突变性,并已被国际癌症研究组织列为Ⅰ类致癌化合物[4-5]。
AFM1在乳及乳制品中的污染情况时有发生。研究发现,非洲、亚洲及欧洲的生鲜乳中均存在AFM1浓度较高(>0.5 μg/kg)的情况[6]。调查我国牛奶及酸奶污染情况发现,西北地区牛奶中AFM1含量明显高于东北地区,且2~3岁的儿童AFM1暴露风险较高,估计其日摄入量(estimated daily intake,EDI)在0.23~0.25 ng/kg,对消费者的健康产生了潜在的危害[7-8]。为此,国内外均制定了严格的限量标准。欧盟限定乳及乳制品中的AFM1不得超过0.05 μg/kg,婴幼儿乳品中不得超过0.025 μg/kg[9]。澳大利亚规定巴氏杀菌婴幼儿牛奶中AFM1不得超过0.01 μg/kg[10]。我国国标GB 2761—2017《食品安全国家标准 食品中真菌毒素限量》规定,乳及婴幼儿乳粉中AFM1不得超过0.5 μg/kg。因此,迫切需要开发高灵敏度、高特异性和快速便捷的检测技术检测乳及乳制品中的AFM1。
生物传感器主要是由分子识别元件及信号转换元件组成,通过信号转换元件将目标物与分子识别元件相互作用转换为可以测量的信号,从而实现对目标物定量分析[11]。因其便携、灵敏、智能等特点受到广泛关注,在真菌毒素检测中迅速发展[12-16]。然而,食品基质复杂多样,对生物传感器灵敏度、准确度等性能提出了更高要求。为此,科研人员将纳米材料信号放大、生物分子信号放大等策略应用于生物传感器中[17-19]。如图1所示,本文综述了近年来基于信号放大策略构建AFM1生物传感器的研究进展,探讨信号放大策略在生物传感器应用中的优势及局限,并对其发展趋势进行展望。
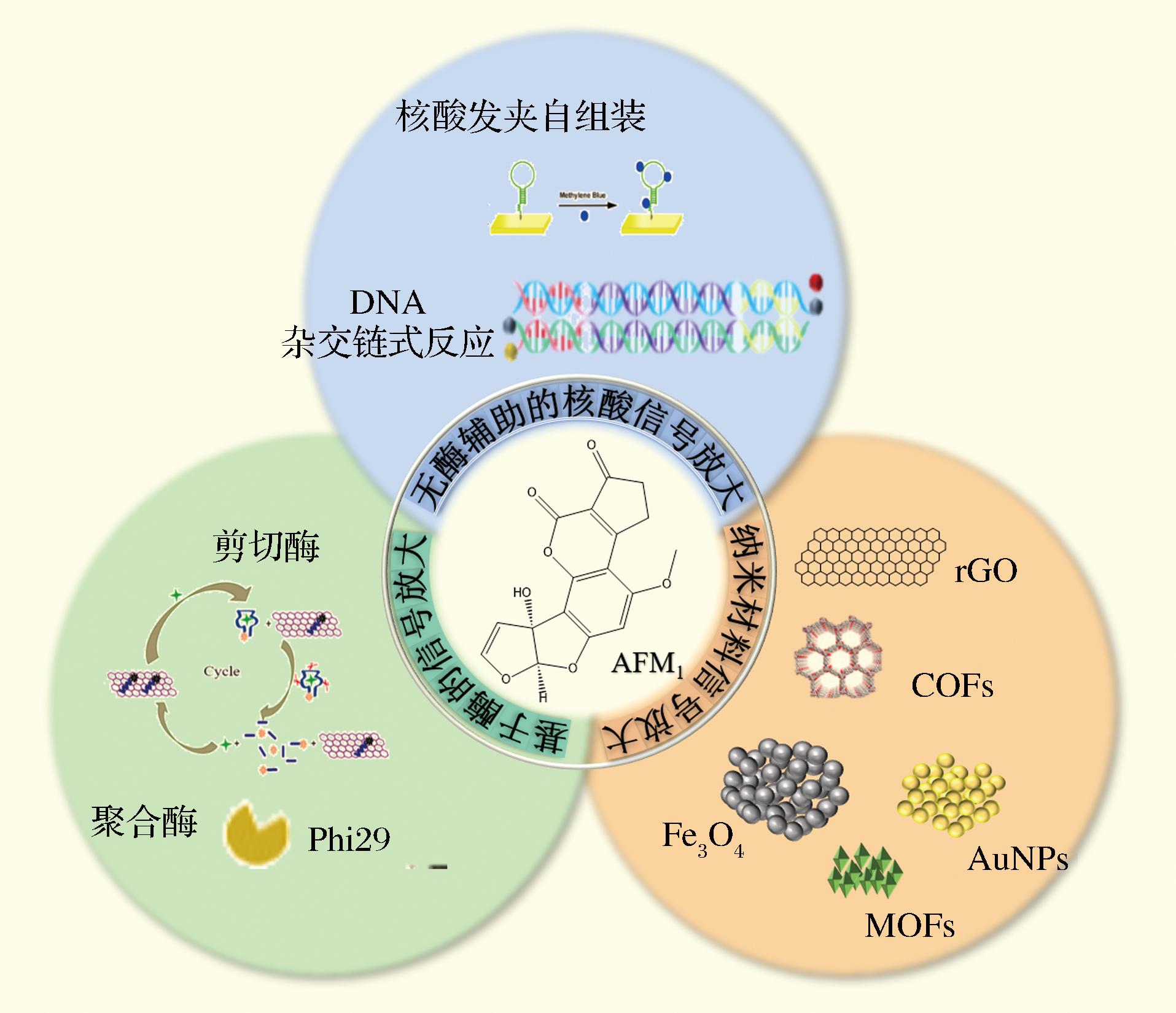
图1 信号放大策略在AFM1生物传感器中的应用
Fig.1 Application of signal amplification strategy in AFM1 biosensor
1 基于纳米材料的信号放大策略
1.1 纳米材料信号放大技术在电化学生物传感器中的应用
电化学生物传感器是将生物分子之间的特异性识别作用转化成电信号,从而实现对目标物的定性或定量检测[20]。纳米材料因其高比表面积、良好催化性能及高导电性能等优势,不仅可以提供较高的电化学检测信号,还可以作为识别元件载体,从而提高电化学生物传感器的检测灵敏度。目前,氧化石墨烯、金纳米颗粒、磁性纳米材料等纳米材料已经成功用于AFM1检测[21]。
基于电子阻碍效应的电化学生物传感器,是由于识别目标物AFM1后电极表面电子转移受到阻碍,电信号发生相应变化,从而实现AFM1的检测[22-25]。AHMADI等[26]以石墨电极为工作电极构建适配体电化学传感器。采用电沉积法制备还原氧化石墨烯(reduced graphene oxided,rGO)与金纳米颗粒(gold nanoparticles,AuNPs)修饰工作电极。适配体通过Au-S固定在AuNPs表面,而rGO比表面积大,能够负载更多AuNPs,可以促进电子转移,产生较高的电信号。当体系中存在AFM1时,适配体与AFM1的特异性结合会阻碍电极表面的电子传递,通过测定电信号变化实现AFM1定量分析,检测限低至0.3 ng/L。KORDASHT等[27]基于新型生物相容性材料建立电化学传感器实现对AFM1的特异性检测,如图2-a所示。采用微波水热法合成氨基功能化的树状纤维纳米二氧化硅(KCC-1-nPr-NH2),再通过电沉积法将其与壳聚糖负载的金纳米颗粒沉积在电极表面,并将标记有信号物的适配体固定在电极表面,AFM1与适配体结合阻碍电子传递使电信号发生变化从而实现对AFM1高灵敏度的检测。YANG等[28]构建了双识别电化学生物传感器用于牛奶中AFM1灵敏检测(图2-b)。采用水热法制备金属有机框架材料(MIL-101),利用适配体互补链建立基于MIL-101的电化学探针(cApt-Au@PEIM)。利用金沉积和电聚合法制备适配体-分子印迹聚合物修饰的工作电极,当目标AFM1缺失时,暴露在印迹层外部的适配体可以通过碱基互补配对与cApt-Au@PEIM探针结合,并表现出强烈的信号响应;当存在AFM1时,其可以与适配体结合并填充分子印迹聚合物的空腔,探针从电极表面释放,电信号被大大减弱。NGUYEN等[29]将磁性纳米颗粒Fe3O4掺杂聚苯胺(polyaniline,PANI)的薄膜聚合在叉指电极(interdigital electrode,IDE)上,如图2-c所示,固定化适配体作为分子识别元件,并将磁性纳米颗粒作为信号放大元件构建电化学生物传感器,通过循环伏安法和方波伏安法的监测电信号变化,检测限低至1.98 ng/L。

a-基于KCC-1-nPr-NH2和壳聚糖负载的AuNPs构建的AFM1电化学适配体传感器[27];b-基于cApt-Au@PEIM构建适配体传感器检测AFM1[28];c-基于磁性纳米颗粒Fe3O4和PANI构建传感器检测AFM1[29]
图2 基于电子阻碍效应的电化学生物传感器检测AFM1
Fig.2 Electrochemical biosensor detection of AFM1 based on electron hindrance effect
基于电信号活性物质的生物传感器是依赖识别过程导致电信号标记物电子转移变化而实现目标物测定[30-31]。GUO等[32]采用原位生长法在电极表面制备共价有机骨架(covalent organic frameworks,COFs),构建电化学传感平台。利用酰胺反应将AFM1适配体固定在磁性纳米颗粒Fe3O4表面,二茂铁标记的适配体互补链(Fc-cDNA)与适配体杂交形成双链DNA。体系中存在AFM1时,目标物与适配体结合,Fc-cDNA释放,基于磁性纳米颗粒Fe3O4得到含有互补链的上清液,并将其滴加在电化学传感平台,以差分脉冲伏安法测定二茂铁的信号变化,从而实现对AFM1的定量检测,如图3-a所示。HAMAMI等[33]利用AuNPs修饰的丝网印刷碳电极为工作电极,将四乙二醇二茂铁(ferrocene tetraethylene glycol ligand,FcTGL)和适配体层层组装在电极表面构建电化学检测平台(图3-b)。AFM1与适配体结合使FcTGL产生电容信号,从而实现对AFM1的高灵敏特异性检测。KULIKOVA等[34]通过电沉积法将PANI固定在电极表面,并将适配体包埋在PANI薄膜之间,随着适配体与AFM1结合,PANI的氧化还原活性显著降低而使电信号发生变化,构建基于PANI双层夹心结构的电化学生物传感器。
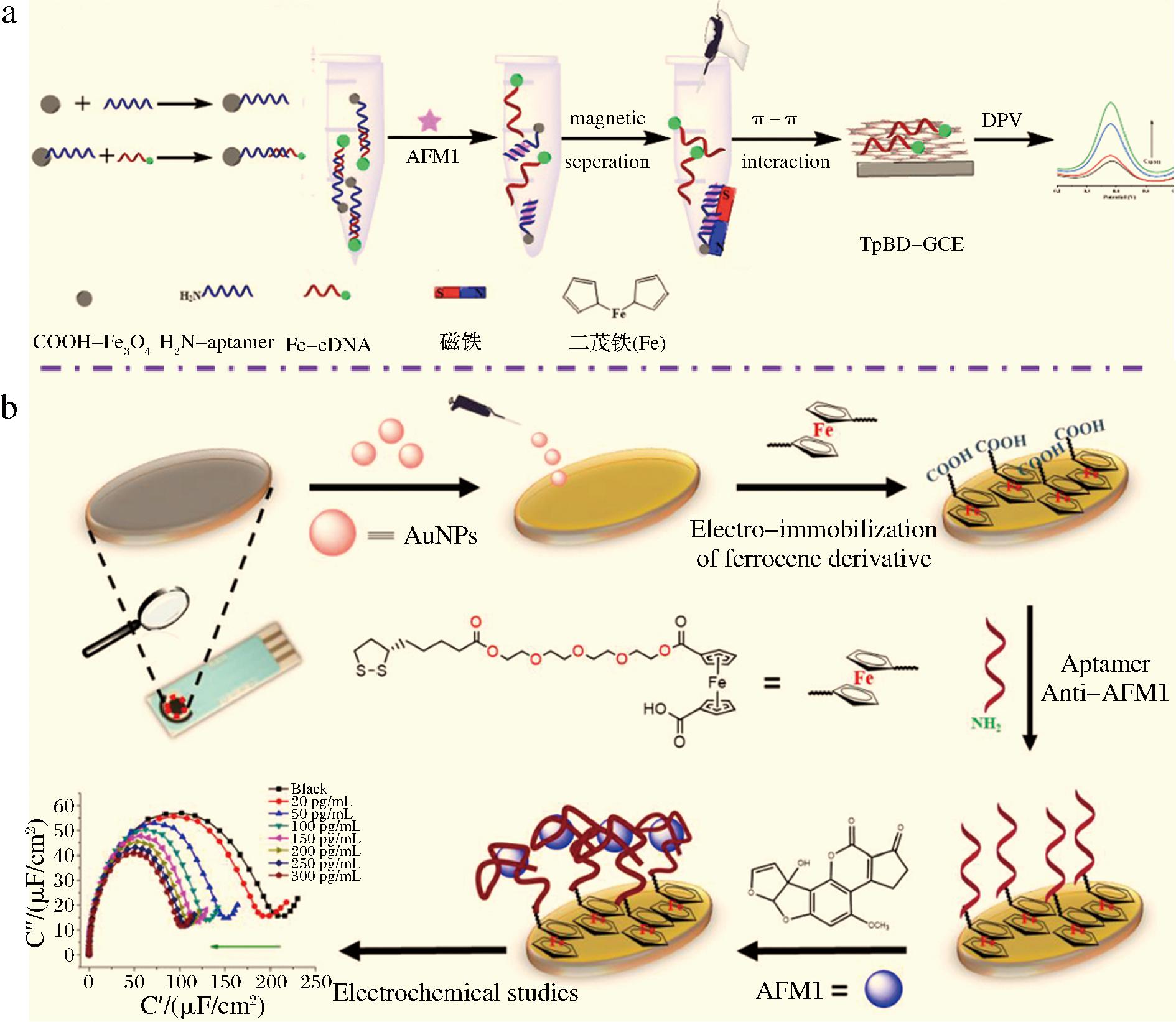
a-基于AFM1和Fc-cDNA对适配体的竞争效应构建生物传感器检测AFM1[32];b-AFM1/FcTGL/AuNPs/SPCE适配体传感器构建[33]
图3 基于电信号活性物质的电化学生物传感器检测AFM1
Fig.3 Electrochemical biosensor detection of AFM1 based on electrical signal active species
1.2 纳米材料信号放大技术在荧光生物传感器中的应用
荧光生物传感器是以荧光物质为传感元件,通过荧光强度变化实现目标物定量分析,具有操作简便、灵敏度高、快速精确和高选择性等优势[35]。纳米材料作为信号放大的有效技术,在荧光传感器中根据作用方式主要分为两类[36-37]:一类是间接信号放大技术,即纳米材料作为荧光猝灭剂,通过抑制背景信号来提高信噪比;二是直接信号放大技术,纳米材料作为固定分子识别元件的载体,将生物信号转换为荧光信号,从而达到检测AFM1的目的。
二硫化钼、金纳米颗粒及石墨烯材料等作为间接信号放大技术中常用的纳米材料,被应用于AFM1的荧光生物传感器中。LI等[38]利用羧基荧光素(5-carboxyfluorescein,FAM)标记适配体与钯纳米粒子(palladium nanoparticles,PdNPs)之间的荧光猝灭作用,构建了一种检测AFM1的高灵敏度荧光适配体传感器。基于相同原理,成功采用FAM及Fe3O4磁性纳米颗粒制备荧光生物传感器[39]。然而,此类生物传感器需要对适配体进行信号标记,不仅成本高,而且会影响适配体的识别效果。为了进一步提高灵敏度,科研人员引入适配体互补链。SAMEIYAN等[40]构建基于二硫化钼纳米片的二价结合适配体-互补链DNA(BBA-cDNA)荧光传感器检测AFM1。FAM标记的适配体互补链作为荧光探针,与适配体形成BBA-cDNA,并通过π-π堆积作用吸附在二硫化钼表面,二硫化钼作为荧光猝灭剂,造成荧光强度降低。加入AFM1后,适配体识别AFM1造成BBA-cDNA构象变化,从二硫化钼表面脱除,荧光强度恢复,依据荧光变化对AFM1定量分析。此荧光传感器中适配体未修饰标记物,具有较高的结构稳定性和灵敏度。
直接信号放大技术将纳米材料作为固定荧光探针的载体,与传统的荧光染料相比,荧光纳米材料具有形貌尺寸可控、易于修饰等优势[41-43],在AFM1荧光传感器中应用广泛。SU等[44]采用聚乙烯亚胺(polyethylenimine,PEI)和聚对苯乙烯磺酸钠(poly sodium-p-styrenesulfonate,PSS)制备红色二氧化硅纳米颗粒,将AFM1单克隆抗体(monoclonal antibody,mAb)固定在此二氧化硅纳米材料上作为信号放大探针,建立竞争性免疫层析法对AFM1进行高灵敏度检测,如图4-a所示。SINGH等[45]以柠檬酸和聚乙烯亚胺为前驱体制备氮掺杂氮掺杂碳量子点(carbon quantum dots,CQDs),基于偶联反应将AFM1抗体固定在CQDs表面得到荧光探针,将合成的探针固定在试纸条上,抗体与AFM1结合,造成荧光强度降低,实现AFM1特异性检测(图4-b)。WANG等[46]合成新型双功能磁-荧光复合纳米球,结合侧向流免疫分析技术,利用荧光识别实现对AFM1的可视化定量检测,如图4-c所示。AFM1存在时,检测线(T线)可以显示从红色荧光到绿色荧光的颜色变化,实现半定量分析,并可以借助智能手机设备结合RGB分析进行准确的定量检测。负载绿色荧光CdZnSe/CdS/ZnS量子点(gSQS)的二氧化硅纳米球与AFM1抗原结合固定在T线,产生绿色荧光作为参照。以Fe3O4为核制备核壳磁性二氧化硅纳米球,负载红色荧光CdSe/CdS/ZnS量子点(RQD)后,修饰AFM1抗体(FSQS-mAbs)。此复合材料与牛奶样品中AFM1结合利用磁性分离实现AFM1预浓缩。如图4-c所示,不存在AFM1时,FSQS-mAbs与T线上AFM1结合,FSQS发射的红色荧光和gSQS发射的绿色荧光重叠,最终显示红色。体系中加入AFM1后,一定量的FSQs-mAbs与AFM1结合,导致与T线结合的FSQs-mAb变少,显示出绿色荧光。
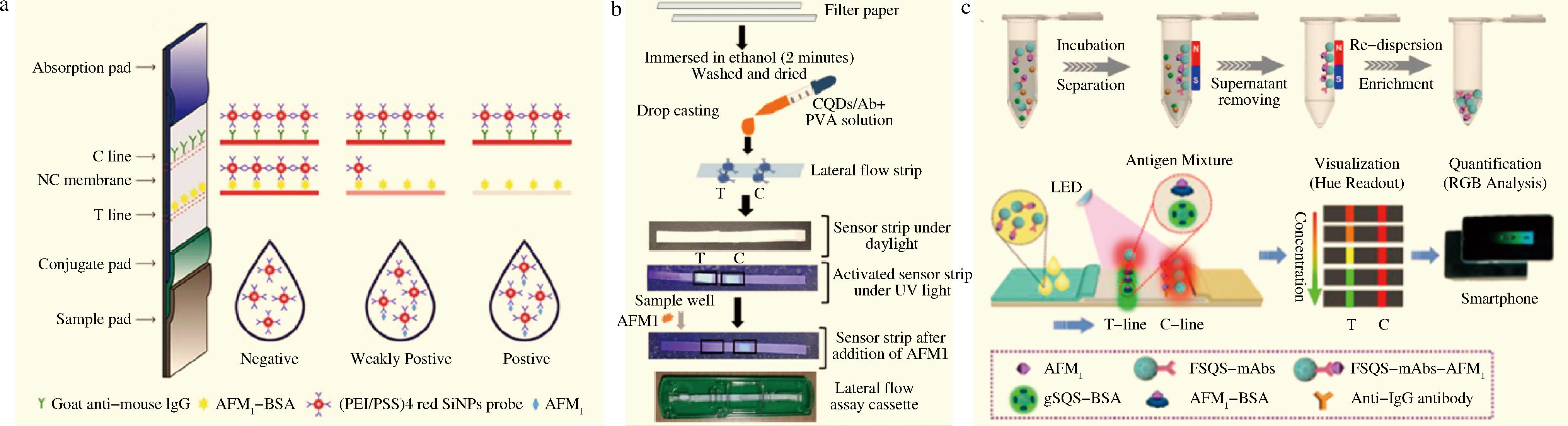
a-基于(PEI/PSS)4的免疫层析竞争检测AFM1的原理图[44];b-基于CQDs/Ab侧向流免疫分析技术的检测原理[45];c-智能手机设备通过RGB颜色分析定量检测AFM1的原理图[46]
图4 直接信号放大策略在AFM1荧光传感器中的应用
Fig.4 Application of direct signal amplification strategy in AFM1 fluorescence sensor
1.3 纳米材料信号放大技术在比色生物传感器中的应用
比色生物传感器指通过目标物引起体系颜色的变化进行定量或定性分析,因其具有制备简单、结果直观的优点,在快速检测领域具有较大的应用前景[47]。基于AuNPs聚集原理是构建比色生物传感器最常见的形式之一[48-49]。LERDSRI等[50]以高特异亲和72-Mers ssDNA作为生物识别元件,基于NaCl诱导AuNPs聚集效应构建比色生物传感器,在适配体的作用下,可以避免 AuNPs在溶液中聚集,AFM1加入溶液中,适配体与AFM1特异性结合,NaCl溶液中AuNPs聚集引起颜色变化,如图5-a所示。基于相同原理,JALALIAN等[51]利用二氧化硅和适配体互补链构建AFM1比色适配体传感器。

a-基于AuNPs聚集的AFM1比色适配体传感器[50];b-基于纳米酶的比色传感器[55];c-AFM1比色生物传感器[56]
图5 纳米材料在AFM1比色生物传感器中的应用
Fig.5 Application of nanomaterials in AFM1 colorimetric biosensors
纳米材料具有类过氧化酶活性,因此,基于纳米酶的比色生物传感器也受到广泛关注[52-54]。WEI等[55]基于AuNPs@CuO复合材料构建比色传感器测定AFM1。为了提高适配体结合量,并且增加纳米材料催化特性,在花状CuO表面固定AuNPs形成AuNPs@CuO复合纳米材料,利用Au-S将适配体固定在复合材料表面,并与修饰在磁珠表面的适配体互补链杂交后形成复合探针。如图5-b所示,存在AFM1时,适配体识别目标物,双链解开,通过磁分离获得上清液,在其中加入3,3′,5,5′-四甲基联苯胺(3,3′,5,5′-tetramethylbenzidine,TMB)显色液,TMB在H2O2作用下被上清液中游离的AuNPs@CuO催化氧化,发生颜色变化,实现AFM1检测。ESMAELPOURFARKHANI等[56]基于CRISPR-Cas12a技术与MnO2的氧化酶活性构建用于AFM1检测的比色适配体传感器(图5-c)。AFM1不存在时,MnO2的氧化酶特性使TMB被氧化,溶液呈黄色;当体系中加入AFM1后,CRISPR-Cas12a无法特异性切割ssDNA,MnO2氧化酶活性被ssDNA降低,无法氧化TMB,溶液呈现浅黄色。
2 基于生物分子的信号放大技术
基于生物分子的信号放大技术主要分为基于酶的信号放大技术与无酶辅助的核酸信号放大技术,酶信号放大技术是通过酶的催化作用实现信号放大,其中最常用的两种酶是核酸酶及聚合酶[57]。核酸酶辅助的信号放大技术是指通过核酸酶对核酸的循环消化和切割实现信号放大[58-59]。GUO等[60]以FAM标记AFM1适配体为荧光探针,石墨烯为荧光猝灭剂,AFM1存在时适配体从石墨烯表面脱离,并与AFM1结合,借助DNA酶I的作用,将与AFM1结合的适配体水解成短片残基,AFM1被释放进入新的循环,从而达到放大信号的作用,基于此构建荧光适配体传感器对AFM1进行检测(图6-a)。ZHANG等[61]将适配体固定在磁性纳米颗粒上,并引入互补链与适配体结合形成双链DNA,如图6-b所示,体系中加入AFM1后,cDNA从体系中释放,在自催化核酸外切酶Ⅲ(exo Ⅲ)作用下产生G-四链体结构,并与荧光染料N-甲基卟啉二丙酸IX结合使荧光信号放大,基于酶信号放大技术构建AFM1高灵敏生物传感器。
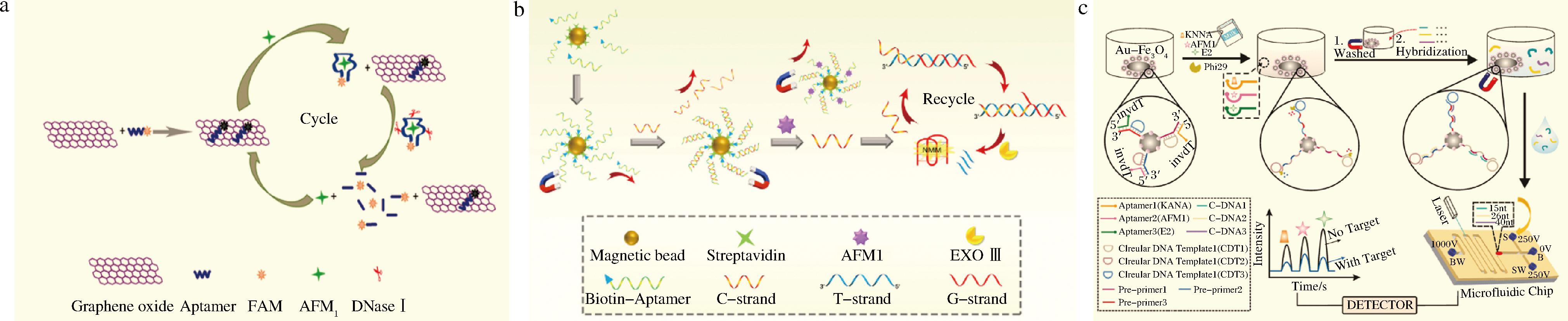
a-荧光生物传感器检测AFM1[60];b-基于剪切酶Exo Ⅲ实现循环信号放大的原理示意图[61];c-同时测定牛奶中卡那霉素、AFM1和雌二醇的传感器示意图[65]
图6 基于酶的信号放大策略在生物传感器中的应用
Fig.6 Application of enzyme-based signal amplification strategies in biosensors
聚合酶辅助的信号放大策略是在聚合酶的作用下短时间内对核酸进行扩增来实现信号放大[62-64]。HE等[65]构建基于磁性材料功能化适配体和微流控芯片的生物传感器同时检测AFM1、卡那霉素和雌二醇。如图6-c所示,前引物-环状DNA模板-适配体组装固定在Au-Fe3O4磁性纳米材料表面,加入phi29聚合酶,未添加目标物时,AP-PP阻碍了phi29聚合酶催化作用,滚环扩增(rolling circle amplification,RCA)无法启动。添加目标物后,适配体与目标物作用,从DNA自组装复合物释放,前引物的3′段暴露,前引物作为引物启动RCA,磁性分离后,将磁性探针加入含有PCA产物互补链的溶液中,造成产物互补链浓度降低,其所对应的还原峰降低。为了进一步提高灵敏度,科研人员将酶信号放大与纳米材料信号放大相结合,实现多策略的信号放大。PANG等[66]采用引物-AuNPs-适配体作为识别元件,将RCA与共价有机骨架纳米材料(covalent organic frameworks, COFs)偶联,实现级联信号放大,建立了灵敏、高选择性、高通量的AFM1电化学免疫分析方法。然而,酶对反应条件要求相对苛刻。
无酶辅助的核酸信号放大技术对反应条件的要求不严格,是复杂体系中AFM1检测的理想传感技术。主要包括DNA的杂交链式反应以及由核酸发夹自组装形成的核酸的催化组装技术[67-68]。JALALIAN等[69]基于AuNPs和发夹结构适配体构建电化学适配体传感器。如图7-a所示,不存在目标物AFM1时,适配体的发夹结构完好,亚甲基蓝(methylene blue,MB)负载量有限,产生的电流信号较为微弱。AFM1存在时,适配体构象发生改变,AuNPs标记的互补链与其结合,使MB的负载量大大提高,电流信号也随之增强,从而实现对AFM1的定量检测,检出限低至0.9 ng/L。GE等[70]基于荧光能量共振转移实现AFM1和AFB1同时检测。适配体与其互补链杂交导致荧光染料Cy3和Cy5荧光猝灭。加入目标物时,适配体识别目标物,双链打开,荧光恢复,如图7-b所示。
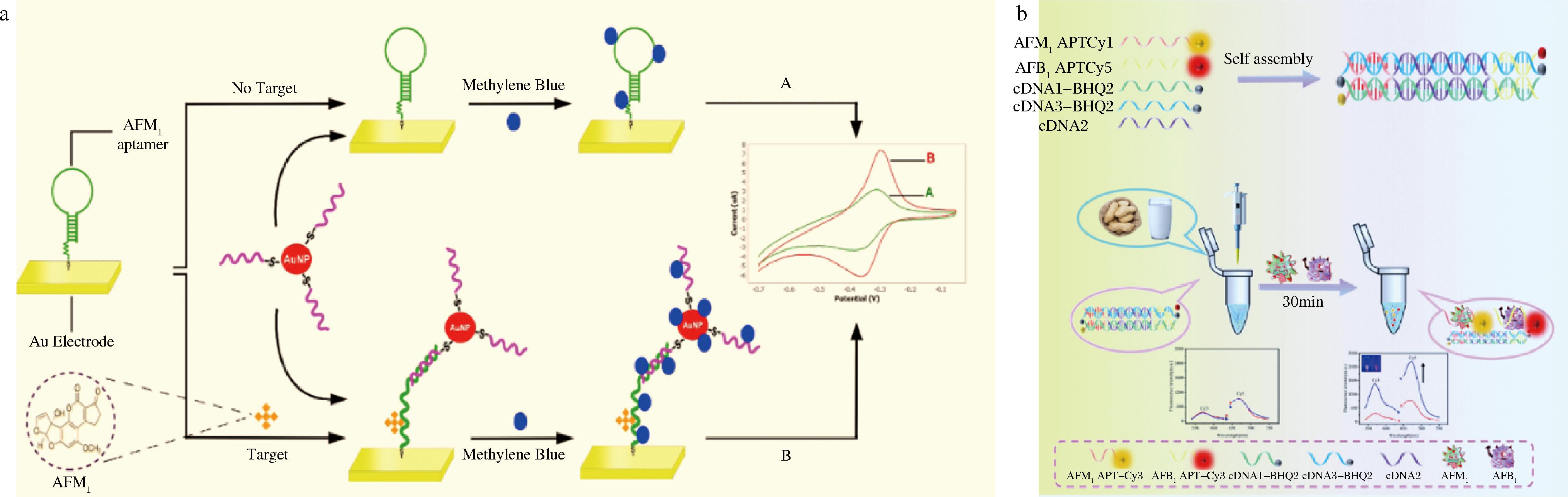
a-基于AuNPs和适配体发夹结构的AFM1电化学传感器[69];b-同时检测AFM1和AFB1的荧光生物传感器[70]
图7 基于酶的信号放大策略在生物传感器中的应用
Fig.7 Application of enzyme-based signal amplification strategies in biosensors
3 结论与讨论
新型生物传感器能够特异性识别目标物,具有高灵敏度、方便快捷的特点,广泛用于AFM1检测,信号放大技术可以进一步提高生物传感器的灵敏度。目前,由于其独特的性质,纳米信号放大技术在AFM1生物传感器中的应用,能够减少非特异性识别,并显著放大传感信号,从而提高其检测灵敏度,但在实际应用中,纳米材料的稳定性与重复性是需要进一步研究的问题,此外,开发低成本、高效的合成方法对于纳米材料的广泛应用也至关重要。此外,基于酶的信号放大技术具有高灵敏度和高特异性,但酶对反应条件要求苛刻,往往需要复杂的操作流程。而无酶辅助的核酸信号放大技术操作简单,成本较低,但在灵敏度和特异性方面存在一定的局限性。
尽管基于信号放大策略的生物传感器在AFM1检测方面取得了显著进展,但仍存在以下问题和挑战:(a)AFM1常污染乳及乳制品,实际样品中存在多种干扰物质,目前多采用磁性纳米材料对其进行富集,但仍需进一步开发在实际样品中直接进行检测的AFM1生物传感器;(b)信号放大技术的稳定性和重复性目前研究较为局限,如何提高传感器的长期稳定性和在不同环境下的重复性,是未来研究的重要方向;(c)信号放大技术提高了检测灵敏度,但部分技术成本较高,限制了其在实际应用中的推广。因此,开发低成本、高效率的信号放大策略,是推动基于信号放大技术的AFM1生物传感器广泛应用的重要途径。
[1] OKECHUKWU V O, ADELUSI O A, KAPPO A P, et al.Aflatoxins:Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques[J].Food Chemistry, 2024, 436:137775.
[2] CAO H H, LIANG D, TANG K Z, et al.SERS and MRS signals engineered dual-mode aptasensor for simultaneous distinguishment of aflatoxin subtypes[J].Journal of Hazardous Materials, 2024, 462:132810.
[3] 李翀. 黄曲霉分生孢子萌发期代谢变化规律及其调控机制研究[D].武汉:华中农业大学, 2022.
LI C.The study on metabolic changes and regulation mechanism of Aspergillus flavus conidia during germination[D].Wuhan:Huazhong Agricultural University, 2022.
[4] 张焕, 高亚男, 郑楠, 等.黄曲霉毒素M1与赭曲霉毒素A联合作用诱导分化Caco-2细胞凋亡的机制[J].中国食品学报, 2019, 19(3):93-101.
ZHANG H, GAO Y N, ZHENG N, et al.Combined effects of aflatoxin M1and ochratoxin A on the apoptosis and mechanism in differentiated Caco-2 Cells[J].Journal of Chinese Institute of Food Science and Technology, 2019, 19(3):93-101.
[5] MIN L, FINK-GREMMELS J, LI D G, et al.An overview of aflatoxin B1 biotransformation and aflatoxin M1 secretion in lactating dairy cows[J].Animal Nutrition, 2021, 7(1):42-48.
[6] MIN L, LI D G, TONG X, et al.The challenges of global occurrence of aflatoxin M1 contamination and the reduction of aflatoxin M1 in milk over the past decade[J].Food Control, 2020, 117:107352.
[7] XIONG J L, WEN D F, ZHOU H L, et al.Occurrence of aflatoxin M1 in yogurt and milk in central-eastern China and the risk of exposure in milk consumers[J].Food Control, 2022, 137:108928.
[8] XU N N, XIAO Y P, XIE Q G, et al.Occurrence of aflatoxin B1 in total mixed rations and aflatoxin M1 in raw and commercial dairy milk in Northern China during winter season[J].Food Control, 2021, 124:107916.
[9] CORDEIRO F, BAER I, ROBOUCH P, et al.Setting maximum limits for trace elements in baby food in European legislation:The outcome of international measurement evaluation programme®-33[J].Food Additives &Contaminants.Part A, Chemistry, Analysis, Control, Exposure &Risk Assessment, 2013, 30(4):678-686.
[10] PECORELLI I, GUARDUCCI N, VON HOLST C, et al.Critical comparison of analytical performances of two immunoassay methods for rapid detection of aflatoxin M1 in milk[J].Toxins, 2020, 12(4):270.
[11] SHARMA P, PANDEY V, SHARMA M M M, et al.A review on biosensors and nanosensors application in agroecosystems[J].Nanoscale Research Letters, 2021, 16(1):136.
[12] LI R X, WEN Y, WANG F L, et al.Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods[J].Journal of Animal Science and Biotechnology, 2021, 12(1):108.
[13] SUN X Y, SUN J D, YE Y L, et al.Metabolic pathway-based self-assembled Au@MXene liver microsome electrochemical biosensor for rapid screening of aflatoxin B1[J].Bioelectrochemistry, 2023, 151:108378.
[14] CUI H N, AN K Q, WANG C Q, et al.A disposable ratiometric electrochemical aptasensor with exonuclease I-powered target recycling amplification for highly sensitive detection of aflatoxin B1[J].Sensors and Actuators B:Chemical, 2022, 355:131238.
[15] SUN C N, LIAO X F, HUANG P X, et al.A self-assembled electrochemical immunosensor for ultra-sensitive detection of ochratoxin A in medicinal and edible malt[J].Food Chemistry, 2020, 315:126289.
[16] RADI A E, EISSA A, WAHDAN T.Molecularly imprinted impedimetric sensor for determination of mycotoxin Zearalenone[J].Electroanalysis, 2020, 32(8):1788-1794.
[17] CINGOLANI M, MUMMOLO L, LUGLI F, et al.Protein aggregation detection with fluorescent macromolecular and nanostructured probes:Challenges and opportunities[J].New Journal of Chemistry, 2021, 45(32):14259-14268.
[18] HUANG F C, ZHANG Y C, LIN J H, et al.Biosensors coupled with signal amplification technology for the detection of pathogenic bacteria:A review[J].Biosensors, 2021, 11(6):190.
[19] WANG S C.Construction of DNA biosensors for mercury (Ⅱ) ion detection based on enzyme-driven signal amplification strategy[J].Biomolecules, 2021, 11(3):399.
[20] SINGH A, SHARMA A, AHMED A, et al.Recent advances in electrochemical biosensors:Applications, challenges, and future scope[J].Biosensors, 2021, 11(9):336.
[21] THURNER F, ALATRAKTCHI F A.Recent advances in electrochemical biosensing of aflatoxin M1 in milk-A mini review[J].Microchemical Journal, 2023, 190:108594.
[22] 惠媛媛, 王毕妮, 张富新, 等.基于还原氧化石墨烯的电化学适配体传感器对黄曲霉毒素M1的检测[J].食品工业科技, 2021, 42(14):249-256.
HUI Y Y, WANG B N, ZHANG F X, et al.An electrochemical aptasensor for detection of aflatoxin M1 based on reduced graphene oxide[J].Science and Technology of Food Industry, 2021, 42(14):249-256.
[23] PANDEY A K, RAJPUT Y S, SHARMA R, et al.Immobilized aptamer on gold electrode senses trace amount of aflatoxin M1[J].Applied Nanoscience, 2017, 7(8):893-903.
[24] ABERA B D, FALCO A, IBBA P, et al.Development of flexible dispense-printed electrochemical immunosensor for aflatoxin M1 detection in milk[J].Sensors, 2019, 19(18):3912.
[25] CHROUDA A, AYED D, ZINOUBI K, et al.Highly stable and ultra-sensitive amperometric aptasensor based on pectin stabilized gold nanoparticles on graphene oxide modified GCE for the detection of aflatoxin M1[J].Food Chemistry Advances, 2022, 1:100068.
[26] AHMADI S F, HOJJATOLESLAMY M, KIANI H, et al.Monitoring of Aflatoxin M1 in milk using a novel electrochemical aptasensor based on reduced graphene oxide and gold nanoparticles[J].Food Chemistry, 2022, 373:131321.
[27] KORDASHT H K, HASANZADEH M.Specific monitoring of aflatoxin M1 in real samples using aptamer binding to DNFS based on turn-on method:A novel biosensor[J].Journal of Molecular Recognition, 2020, 33(6):e2832.
[28] YANG D, HUI Y Y, LIU Y Y, et al.Novel dual-recognition electrochemical biosensor for the sensitive detection of AFM1 in milk[J].Food Chemistry, 2024, 433:137362.
[29] NGUYEN B H, TRAN L D, DO Q P, et al.Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor[J].Materials Science and Engineering:C, 2013, 33(4):2229-2234.
[30] WANG B Z, AKIBA U, ANZAI J I.Recent progress in nanomaterial-based electrochemical biosensors for cancer biomarkers:A review[J].Molecules, 2017, 22(7):1048.
[31] 靖乐. 基于梳型阳离子共聚物介导的DNA电化学生物传感器的构筑及其检测性能研究[D].武汉:中国地质大学, 2023.
JING L.Construction and detection performance of DNA electrochemical biosensor assisted by comb-type cationic copolymer[D].Wuhan:China University of Geosciences, 2023.
[32] GUO L L, WANG Y Y, PANG Y H, et al.In situ growth of covalent organic frameworks TpBD on electrode for electrochemical determination of aflatoxin M1[J].Journal of Electroanalytical Chemistry, 2021, 881:114931.
[33] HAMAMI M, MARS A, RAOUAFI N.Biosensor based on antifouling PEG/Gold nanoparticles composite for sensitive detection of aflatoxin M1 in milk[J].Microchemical Journal, 2021, 165:106102.
[34] KULIKOVA T N, PORFIREVA A V, EVTUGYN G A, et al.Electrochemical aptasensor with layer-by-layer deposited polyaniline for aflatoxin M1 voltammetric determination[J].Electroanalysis, 2019, 31(10):1913-1924.
[35] ZHANG M K, GUO X D.Emerging strategies in fluorescent aptasensor toward food hazard aflatoxins detection[J].Trends in Food Science &Technology, 2022, 129:621-633.
[36] 张小凡. 基于DNA链置换策略的荧光传感分析方法及性能研究[D].青岛:青岛科技大学, 2023.
ZHANG X F.Study on fluorescence sensing analysis method and performance based on DNA strand displacement strategy[D].Qingdao:Qingdao University of Science &Technology, 2023.
[37] 李新. 基于toehold链置换辅助目标循环放大的新型荧光生物传感器的研究[D].重庆:西南大学, 2017.
LI X.Study on a novel fluorescence biosensor based on toehold strand displacement reactions aided target recycling amplification[D].Chongqing:Southwest University, 2017.
[38] LI H, YANG D B, LI P W, et al.Palladium nanoparticles-based fluorescence resonance energy transfer aptasensor for highly sensitive detection of aflatoxin M1 in milk[J].Toxins, 2017, 9(10):318.
[39] 郭婷, 林淑凤, 马良, 等.基于磁性纳米材料和适配体的荧光传感器检测牛奶中黄曲霉毒素M1[J].食品与发酵工业, 2019, 45(5):218-223.
GUO T, LIN S F, MA L, et al.A fluorescent biosensor based on magnetic nanoparticles and aptamer for detecting AFM1 in milk[J].Food and Fermentation Industries, 2019, 45(5):218-223.
[40] SAMEIYAN E, KHOSHBIN Z, LAVAEE P, et al.A bivalent binding aptamer-cDNA on MoS2 nanosheets based fluorescent aptasensor for detection of aflatoxin M1[J].Talanta, 2021, 235:122779.
[41] 蒲源, 王丹, 钱骏, 等.荧光纳米材料及其生物成像应用[J].中国材料进展, 2017, 36(2):103-111.
PU Y, WANG D, QIAN J, et al.Fluorescent nanomaterials and their applications in bioimaging[J].Materials China, 2017, 36(2):103-111.
[42] SUN J D, LI M, XING F G, et al.Novel dual immunochromatographic test strip based on double antibodies and biotin-streptavidin system for simultaneous sensitive detection of aflatoxin M1 and ochratoxin A in milk[J].Food Chemistry, 2022, 375:131682.
[43] FORCADA S, S NCHEZ-VISEDO A, MELENDRERAS C, et al.Design and evaluation of a competitive phosphorescent immunosensor for aflatoxin M1 quantification in milk samples using Mn:ZnS quantum dots as antibody tags[J].Chemosensors, 2022, 10(2):41.
NCHEZ-VISEDO A, MELENDRERAS C, et al.Design and evaluation of a competitive phosphorescent immunosensor for aflatoxin M1 quantification in milk samples using Mn:ZnS quantum dots as antibody tags[J].Chemosensors, 2022, 10(2):41.
[44] SU Z X, ZHAO G Y, DOU W C.Determination of trace aflatoxin M1 (AFM1) residue in milk by an immunochromatographic assay based on (PEI/PSS)4 red silica nanoparticles[J].Mikrochimica Acta, 2020, 187(12):658.
[45] SINGH H, SINGH S, BHARDWAJ S K, et al.Development of carbon quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin M1 in milk[J].Food Chemistry, 2022, 393:133374.
[46] WANG J, JIANG C X, YUAN J R, et al.Hue recognition competitive fluorescent lateral flow immunoassay for aflatoxin M1 detection with improved visual and quantitative performance[J].Analytical Chemistry, 2022, 94(30):10865-10873.
[47] ZHOU J J, LIU Y T, DU X P, et al.Recent advances in design and application of nanomaterials-based colorimetric biosensors for agri-food safety analysis[J].ACS Omega, 2023, 8(49):46346-46361.
[48] SADIQ Z, SAFIABADI TALI S H, HAJIMIRI H, et al.Gold nanoparticles-based colorimetric assays for environmental monitoring and food safety evaluation[J].Critical Reviews in Analytical Chemistry, 2024, 54(7):2209-2244.
[49] 苏柳, 贺伟华, 张干, 等.两种常用适配体的纳米金比色法快速检测牛奶中黄曲霉毒素M1的评价研究[J].食品工业科技, 2024, 45(8):284-292.
SU L, HE W H, ZHANG G, et al.Evaluation of gold nanoparticles colorimetric sensing based on two commonly aptamer for rapid detecting aflatoxin M1 in milk[J].Science and Technology of Food Industry, 2024, 45(8):284-292.
[50] LERDSRI J, SOONGSONG J, LAOLUE P, et al.Reliable colorimetric aptasensor exploiting 72-Mers ssDNA and gold nanoprobes for highly sensitive detection of aflatoxin M1 in milk[J].Journal of Food Composition and Analysis, 2021, 102:103992.
[51] JALALIAN S H, LAVAEE P, RAMEZANI M, et al.An optical aptasensor for aflatoxin M1 detection based on target-induced protection of gold nanoparticles against salt-induced aggregation and silica nanoparticles[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2021, 246:119062.
[52] 刘玉婷, 陆清, 唐志永, 等.基于氧化还原型纳米酶的比色传感器在食品安全检测中的研究进展[J].食品科学, 2024,45(22):311-321.
LIU Y T, LU Q, TANG Z Y, et al.Research progress in colorimetric sensors based on redox-type nanozymes for food safety detection[J].Food Science, 2024,45(22):311-321.
[53] WEI Y, HUANG L H, SHI Z F, et al.Smartphone-integrated colorimetric sensor for rapid and highly selective detection of spermine in food based on the laccase-mimicking activity of flower-shaped Mn3O4 nanoparticles[J].Microchemical Journal, 2024, 198:110148.
[54] QIN S, LIU B, XUE Y T, et al.A three-dimensional network structure of metal-based nanozymes for the construction of colorimetric sensors for the detection of antioxidants[J].Analytical Methods, 2024, 16(15):2292-2300.
[55] WEI X J, MA P F, IMRAN MAHMOOD K, et al.Screening of a high-affinity aptamer for aflatoxin M1 and development of its colorimetric aptasensor[J].Journal of Agricultural and Food Chemistry, 2023, 71(19):7546-7556.
[56] ESMAELPOURFARKHANI M, RAMEZANI M, ALIBOLANDI M, et al.CRISPR-Cas12a-based colorimetric aptasensor for aflatoxin M1 detection based on oxidase-mimicking activity of flower-like MnO2 nanozymes[J].Talanta, 2024, 271:125729.
[57] 席强. 光学生物传感中信号放大策略与石墨烯类纳米材料的应用[D].长沙:湖南大学, 2015.
XI Q.Signal amplification strategies and graphene-type nanomaterials used in optical biosensing technology[D].Changsha:Hunan University, 2015.
[58] MA L, GUO T, PAN S L, et al.A fluorometric aptasensor for patulin based on the use of magnetized graphene oxide and DNase I-assisted target recycling amplification[J].Mikrochimica Acta, 2018, 185(10):487.
[59] 刘萌. 基于酶辅助信号放大的荧光生物传感器的构建及其应用研究[D].济南:山东师范大学, 2021.
LIU M.Construction of enzyme assisted signal a mplification-based fluorescence biosensors for biomedical applications[D].Jinan:Shandong Normal University, 2021.
[60] GUO X D, WEN F, QIAO Q Q, et al.A novel graphene oxide-based aptasensor for amplified fluorescent detection of aflatoxin M1 in milk powder[J].Sensors, 2019, 19(18):3840.
[61] ZHANG F Y, LIU L Y, NI S N, et al.Turn-on fluorescence aptasensor on magnetic nanobeads for aflatoxin M1 detection based on an exonuclease Ⅲ-assisted signal amplification strategy[J].Nanomaterials, 2019, 9(1):104.
[62] WANG S Y, ZONG Z W, XU J G, et al.Recognition-activated primer-mediated exponential rolling circle amplification for signal probe production and ultrasensitive visual detection of ochratoxin A with nucleic acid lateral flow strips[J].Analytical Chemistry, 2023, 95(44):16398-16406.
[63] LONG X Q, WU Q, YANG L, et al.A photothermal aptasensor based on rolling circle amplification-enriched DNAzyme for portable detection of ochratoxin A in grape juice[J].International Journal of Biological Macromolecules, 2024, 269:132279.
[64] ALI M M, LI F, ZHANG Z Q, et al.Rolling circle amplification:A versatile tool for chemical biology, materials science and medicine[J].Chemical Society Reviews, 2014, 43(10):3324-3341.
[65] HE L Y, SHEN Z P, WANG J Q, et al.Simultaneously responsive microfluidic chip aptasensor for determination of kanamycin, aflatoxin M1, and 17β-estradiol based on magnetic tripartite DNA assembly nanostructure probes[J].Mikrochimica Acta, 2020, 187(3):176.
[66] PANG Y H, GUO L L, SHEN X F, et al.Rolling circle amplified DNAzyme followed with covalent organic frameworks:Cascade signal amplification of electrochemical ELISA for alfatoxin M1 sensing[J].Electrochimica Acta, 2020, 341:136055.
[67] 王姝凡, 张雁玲, 张会成, 等.基于信号放大策略和DNA纳米材料的表面增强拉曼生物传感器进展[J].化学传感器, 2021, 41(4):23-32.
WANG S F, ZHANG Y L, ZHANG H C, et al.Research progress in surface-enhanced Raman spectroscopy and biosensor based on signal amplification strategy and DNA nano materials[J].Chemical Sensors, 2021, 41(4):23-32.
[68] GUO T, WANG C C, ZHOU H Y, et al.A facile aptasensor based on polydopamine nanospheres for high-sensitivity sensing of T-2 toxin[J].Analytical Methods, 2021, 13(24):2654-2658.
[69] JALALIAN S H, RAMEZANI M, DANESH N M, et al.A novel electrochemical aptasensor for detection of aflatoxin M1 based on target-induced immobilization of gold nanoparticles on the surface of electrode[J].Biosensors and Bioelectronics, 2018, 117:487-492.
[70] GE G, WANG T L, LIU Z H, et al.A self-assembled DNA double-crossover-based fluorescent aptasensor for highly sensitivity and selectivity in the simultaneous detection of aflatoxin M1 and aflatoxin B1[J].Talanta, 2023, 265:124908.