有机氯农药(organochlorine pesticides, OCPs)是一种公认的持久性有机污染物[1],对生物体的内分泌系统、生殖系统和神经系统均有毒性,可产生致畸、致癌效应[2]。这类物质可通过降雨、地表径流等方式进入水环境[3],同时由于其具有高脂溶性,极易经食物链在生物体内富集并产生生物放大效应[4]。有研究表明,食用鱼类是人体农药摄入的重要途径之一[5],因为它们能通过皮肤和鳃直接从水环境中吸收OCPs,也能通过食物链放大作用富集OCPs[6],其富集系数可达40 000倍[7]。因此,研究鱼类中OCPs的残留量对保障人体健康有重要意义。
鱼肉样品通常含有大量蛋白质和脂肪,基质复杂,对其进行分析时通常需要进行富集和净化等前处理操作。目前,我国关于水产品中有机氯农药测定主要采用GB 23200.88—2016,其中目标物用丙酮-石油醚索氏提取,经弗罗里硅土柱净化后用气相色谱测定,索氏提取需耗费大量溶剂。凝胶渗透色谱[6,8]、硅胶固相萃取[4,9]和磺化[3]在鱼肉样品OCPs测定中运用较多,但凝胶渗透色谱设备昂贵,溶剂耗费较多,固相萃取净化稳定性好,回收率高,但操作效率低下,难以满足大批量样品快速检测的要求,磺化能有效去除鱼肉中的脂肪和蛋白质,但大量硫酸易对操作人员造成身体伤害,且容易污染环境。QuEChERS方法操作简便,回收率好,操作效率高,成功克服了上述样品前处理方法的缺点[10],已广泛应用于水果[11-12]、蔬菜[13-14]、粮食[15-16]和茶叶[17-18]等样品中的农药残留检测,但在鱼类等脂肪含量较高的动物样品中OCPs检测的应用较少。
本试验在国内外标准和文献[10-19,20]的基础上,建立超声波辅助萃取,QuEChERS净化,通过气相色谱-气质联用确证、气相色谱定量测定鱼肉中OCPs的检测方法,并将其用于厦门海域10种鱼类中OCPs的残留分析。
1 材料与方法
1.1 仪器与试剂
7890B气相色谱仪(配电子捕获检测器)、7890B-5977B气相色谱-质谱联用仪(配EISS离子源),美国Agilent公司; 3K15台式高速离心机,德国SIGMA;N-EVAP 12管氮气浓缩仪,上海安谱; T25组织匀浆机、Vortex GENIUS 3涡旋振荡器,德国IKA。
丙酮、正己烷均为色谱纯,美国Fisher Chemical;N-丙基乙二胺(PSA)、C18固相萃取吸附剂,美国Agilent公司;无水硫酸镁、百灵威、α-六六六、β-六六六、γ-六六六、δ-六六六、p,p’-DDE、o,p’-DDT、p,p’-DDD、p,p’-DDT艾氏剂、狄氏剂、七氯、环氧七氯、异狄氏剂、硫丹Ⅰ、硫丹Ⅱ,质量浓度均为100 μg/mL,北京坛墨质检科技有限公司。
1.2 样品的采集与制备
选取从某大型超市购买的青石斑鱼作为方法验证样品,在厦门海域近岸外的9.26 km渔船随机购买10种常见鱼类,采样鱼类相关参数见表1。海冰低温保存带回实验室,每条取可食部分切成小块,混合均匀后制成肉糜,-20 ℃冷冻保存,7 d内完成分析。
表1 采样鱼类相关参数
Table 1 Parameters for fishes
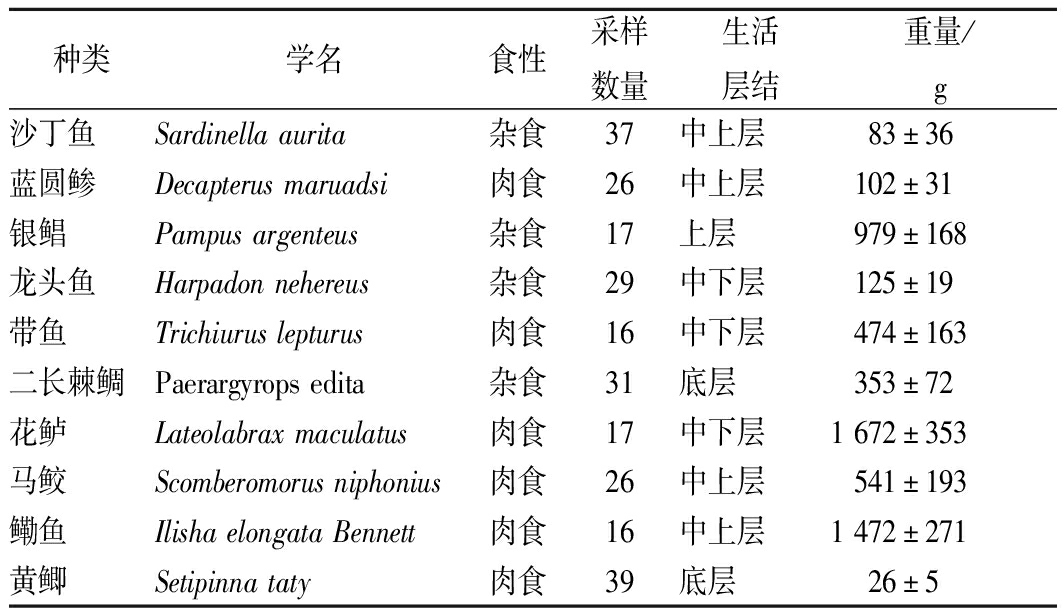
种类学名食性采样数量生活层结重量/g沙丁鱼Sardinella aurita杂食37中上层83±36蓝圆鲹Decapterus maruadsi肉食26中上层102±31 银鲳Pampus argenteus杂食17上层979±168 龙头鱼Harpadon nehereus杂食29中下层125±19 带鱼Trichiurus lepturus肉食16中下层474±163 二长棘鲷Paerargyrops edita杂食31底层353±72 花鲈Lateolabrax maculatus肉食17中下层1 672±353 马鲛Scomberomorus niphonius肉食26中上层541±193 鳓鱼Ilisha elongata Bennett肉食16中上层1 472±271 黄鲫Setipinna taty肉食39底层26±5
1.3 试验方法
1.3.1 样品处理
称取鱼肉样品2.00 g于50 mL离心管,加10 mL V(正己烷)∶V(丙酮)=1∶1,12 000 r/min匀浆提取1 min,加入0.4 g无水MgSO4和0.1 g无水醋酸钠,剧烈振荡1 min,超声10 min,旋涡1 min,8 000 r/min离心2min,移取上清液6 mL于装有400 mg PSA、150 mg C18和900 mg无水MgSO4的15 mL离心管,旋涡1 min,然后以8000 r/min离心2 min,取上清液5 mL于10 mL玻璃刻度试管,40 ℃氮气浓缩近干,用0.1 mL正己烷溶解,旋涡后转移至内置衬管的进样小瓶,用GC-ECD定量,GC-MS确证。
1.3.2 仪器条件
1.3.2.1 气相色谱条件
进样口温度280 ℃,检测器(ECD)温度300 ℃,载气N2流量1.2 mL/min,DB-1MS色谱柱30 m×0.25 mm×0.25 μm,柱初温90 ℃,保持0.5 min,20 ℃/min升温至180 ℃,10 ℃/min升温至230 ℃,20 ℃/min升温至280 ℃,保持6 min,进样量2 μL。
1.3.2.2 气相色谱-质谱联用条件
进样口温度280 ℃,检测器(ECD)温度300 ℃,载气H2流量1.0 mL/min,DB-1MS色谱柱30 m×0.25 mm×0.25 μm,柱初温50 ℃,保持0.5 min,20 ℃/min升温至130 ℃,8 ℃/min升温至250 ℃,15 ℃/min升温至280 ℃,保持5 min,进样量2 μL。SIM采集模式,各组分采集离子参考GB 23200.8—2016。
1.4 方法性能测试
1.4.1 标准曲线
为减小基质效应对定量结果的影响,采用空白基质溶液配制标准曲线系列。分别取各OCPs组分0.1 mL,用正己烷稀释至10.00 mL,配成1.00 μg/mL混合标准溶液,取阴性鱼肉样品按1.3.1处理定容后,用该正己烷溶液将1.00 μg/mL标准溶液稀释成1.00、2.00、5.00、10.00、20.00、50.00 μg/L标准曲线系列,以浓度为横坐标,峰面积为纵坐标绘制标准曲线,外标法定量。
1.4.2 回收率、精密度、检出限和定量限
取鱼肉阴性样品,添加1.00 μg/mL混合标准溶液0.002、0.01和0.05 mL,使样品中各OCPs的浓度为1.0、5.0和10 μg/kg,做加标回收试验,并计算各组分的精密度,每个浓度做6个平行试验。在最低添加浓度(1.0 μg/kg)的样品结果中,以3倍信噪比计算鱼肉样品中各OCPs的检出限,以10倍信噪比计算鱼肉样品中各OCPs的定量限。
1.5 风险评估
人体中所累积的有机污染物大部分来自食物,海产品作为沿海居民的主要食物来源,有必要评估OCPs对人体健康的风险。本试验采用世界卫生组织规定的每日允许摄入量(acceptable daily intake,ADI)作为评价标准,计算居民每日膳食摄入量(estimated daily intake,EDI),与GB 2763—2016中规定的ADI值相比较,评估OCPs对人体健康的潜在风险[21]。具体计算如公式(1)所示:
(1)
式中:ω,鱼肉中OCPs的浓度,μg/kg;CR,鱼类人均每日摄入量,g/d;BW,消费者体质量,kg。
本试验中鱼类人均每日摄入量CR按100 g/d计算,消费者体质量BW采用60 kg。
2 结果与分析
2.1 方法性能试验
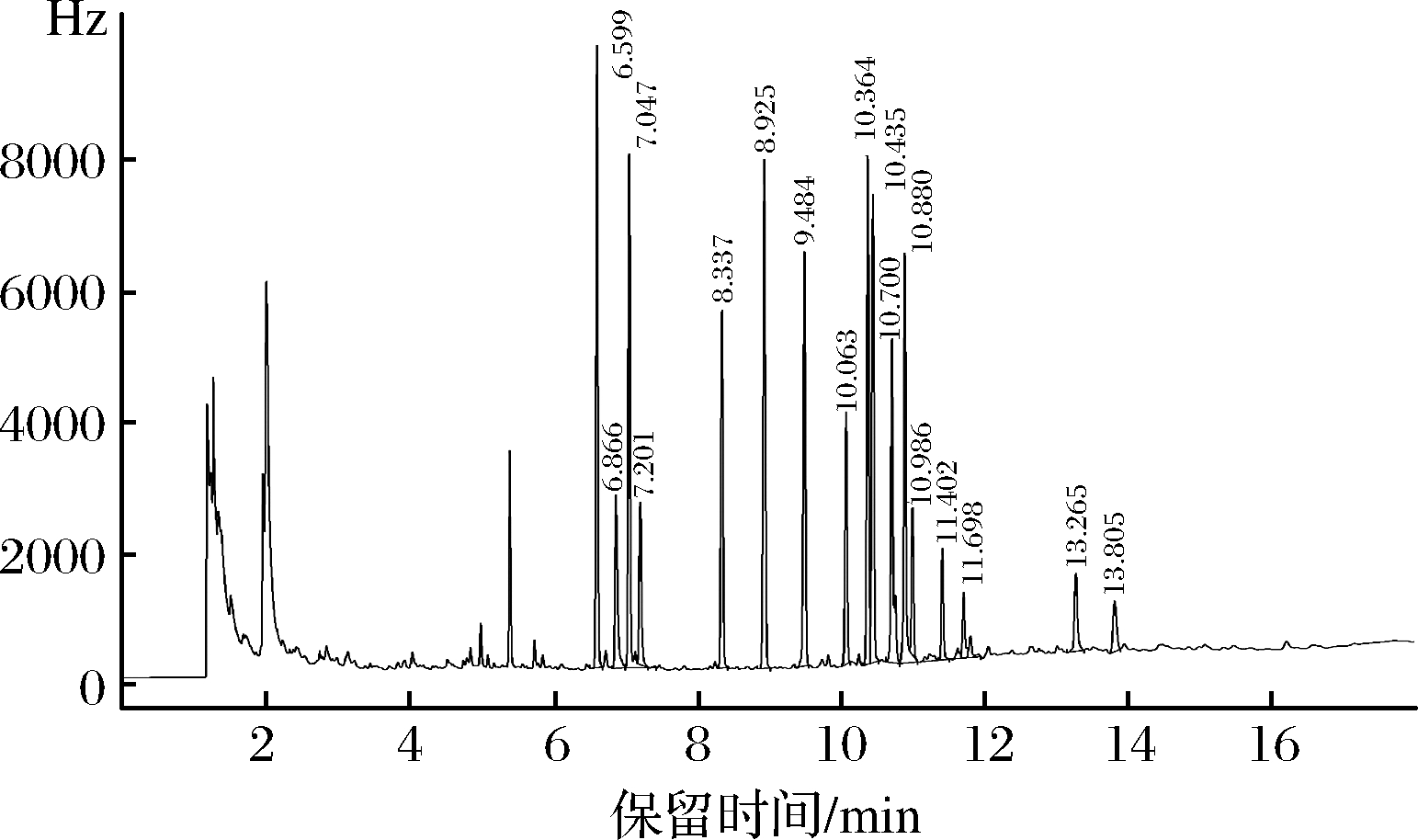
图1 加标样品(0.5 μg/kg)中OCPs的分离色谱图
Fig.1 Gas chromatography of OCPs in fish samples add
standard 0.5 μg/kg
从图1和表2可知,14种OCPs在1.3.2.1的仪器条件下,在1~50 μg/L质量浓度范围内线性关系良好,相关系数均大于0.998 8。鱼肉样品含有15%~20%的蛋白质和5%左右的脂肪,乙腈虽然广泛应用于水果和蔬菜样品的农残提取,但并不适合脂肪含量偏高的样品,因为乙腈会使蛋白质快速变形,阻碍乙腈渗透而导致萃取效率偏低。因此本研究采用GB23200.88—2016相似的萃取溶剂体系提取鱼肉中的OCPs,采用V(正己烷)∶V(丙酮)=1∶1提取OCPs的同时会提取出鱼肉中的脂质,因此减少鱼肉取样量为2 g,同时采用先净化后浓缩的方式,使PSA、C18和MgSO4与溶剂接触更加充分,以获得更好的净化效果。结果表明,经400 mg PSA、150 mg C18和900 mg无水MgSO4净化后,可以基本消除脂质对ECD的影响,同时使用GCMS对阳性样品进行确认,进一步保证结果的准确性。用气相色谱分析1.3.1处理后的加标样品,OCPs的回收率为86.5%~97.4%,精密度为4.8%~12.1%,最低检出限为0.01 μg/kg,能够满足鱼肉中的OCPs分析。
表2 有机氯农药的线性方程、相关系数、回收率、精密度、检出限和定量限
Table 2 The linear equations, correlation coefficients, recovery, precision, LOD and LOQ of OCPs
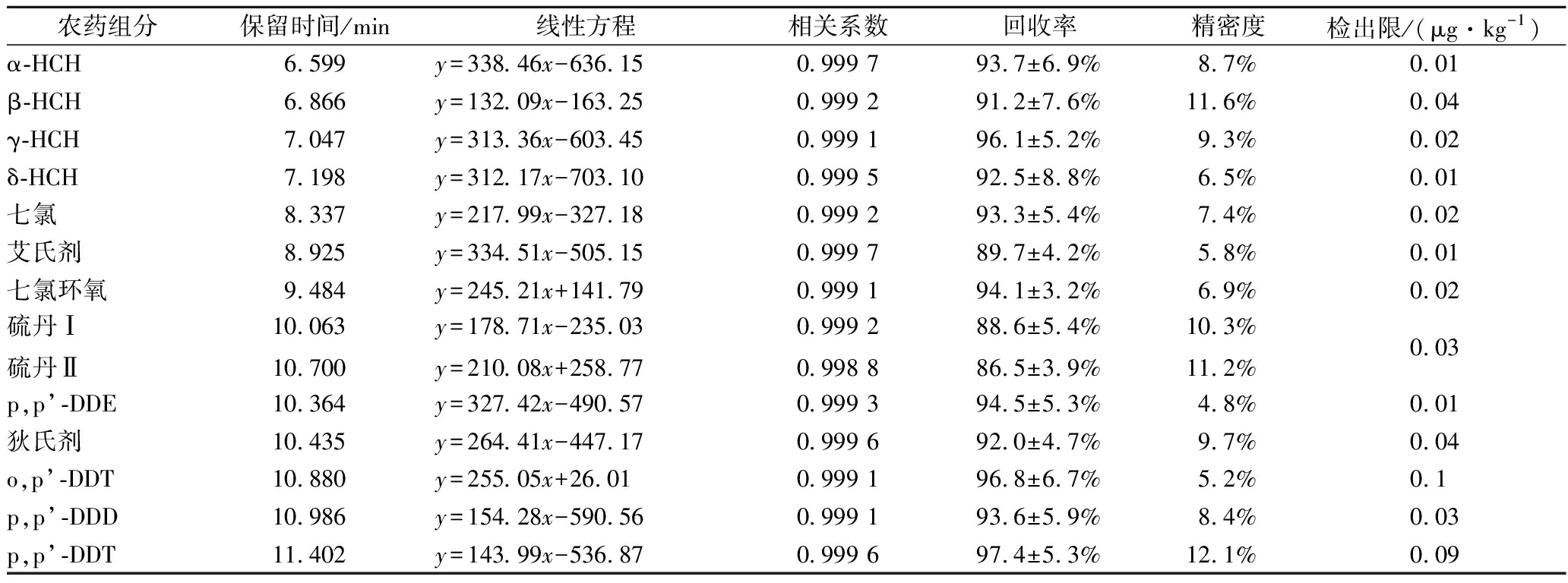
农药组分保留时间/min线性方程相关系数回收率精密度检出限/(μg·kg-1)α-HCH6.599y=338.46x-636.150.999 793.7±6.9%8.7%0.01β-HCH6.866y=132.09x-163.250.999 291.2±7.6%11.6%0.04γ-HCH7.047y=313.36x-603.450.999 196.1±5.2%9.3%0.02δ-HCH7.198y=312.17x-703.100.999 592.5±8.8%6.5%0.01七氯8.337y=217.99x-327.180.999 293.3±5.4%7.4%0.02艾氏剂8.925y=334.51x-505.150.999 789.7±4.2%5.8%0.01七氯环氧9.484y=245.21x+141.790.999 194.1±3.2%6.9%0.02硫丹Ⅰ硫丹Ⅱ10.06310.700y=178.71x-235.03y=210.08x+258.770.999 20.998 888.6±5.4%86.5±3.9%10.3%11.2%0.03p,p’-DDE10.364y=327.42x-490.570.999 394.5±5.3%4.8%0.01狄氏剂10.435y=264.41x-447.170.999 692.0±4.7%9.7%0.04o,p’-DDT10.880y=255.05x+26.010.999 196.8±6.7%5.2%0.1p,p’-DDD10.986y=154.28x-590.560.999 193.6±5.9%8.4%0.03p,p’-DDT11.402y=143.99x-536.870.999 697.4±5.3%12.1%0.09
2.2 实际样品有机氯农药分析及风险评价
OCPs具有疏水性,对脂质具有很高的亲和力,水环境中OCPs的生物富集效应主要依靠水-脂的两相分配[22],鱼类能够直接从水中吸收OCPs,也能通过摄食富集OCPs,但是对OCPs的代谢缓慢,所以常被用于水环境的整体OCPs污染水平评价[23]。
2.2.1 六六六
HCHs曾经作为一种广谱农药而广泛应用于农业,我国自1992年开始禁止使用六六六。工业的HCHs由4种异构体组成,α-HCH(60%~70%)、β-HCH(5%~12%)、γ-HCH(10%~15%)、δ-HCH(6%~10%),而林丹中的γ-HCH超过90%[24-25]。从表3可知,有3种鱼类均检出4种HCHs异构体,这3种鱼类均为肉食性中下层鱼类。黄鲫和带鱼的HCHs含量最高,这可能是黄鲫为常栖息于淤泥底质中,而带鱼脂肪含量较高的原因。所有异构体中β-HCH的检出率和平均浓度最高,α-HCH的检出率最低。这可能是因为在4种异构体中,β-HCH最容易被底层淤泥富集[26],且α-HCH和γ-HCH也可以转化成β-HCH[27];另外,由于β-HCH中氯原子的特殊位置,使其更难被降解[28],导致β-HCH在沉积物中积累,然后在鱼类等水生生物中富集。α-HCH/γ-HCH的值可以用来分析HCHs的来源,当α-HCH/γ-HCH≥3时,表示水环境中的HCHs主要来自工业污染、大气转移和沉积物的输入[29-30],α-HCH /γ-HCH≤1时则表示污染来自于林丹[31-32]。从表3可以看出,10种鱼类中4种HCHs异构体残留的浓度β-HCH>γ-HCH>δ-HCH>α-HCH,所有鱼类中α-HCH与γ-HCH的比值均小于1,表明该水域中HCHs的污染来自于林丹。另外,本研究中的HCHs总量在ND~5.759 μg/kg,黄鲫和带鱼的EDI值最高,分别为5.759和5.665 μg/kg。
表3 鱼类六六六农药含量
Table 3 Levels of HCHs in fish

样品名称α-HCH/(μg·kg-1)β-HCH/(μg·kg-1)γ-HCH/(μg·kg-1)δ-HCH/(μg·kg-1)∑HCHs/(μg·kg-1)EDI/(ng·kg-1 BW)沙丁鱼ND0.034 6NDND0.0450.074蓝圆鲹ND1.732NDND1.7322.887银鲳ND3.2670.0270.019 73.3145.523龙头鱼NDNDNDNDND带鱼ND5.7320.027 3ND 5.7599.599二长棘鲷ND1.147NDND1.1471.912花鲈0.024 63.3280.031 20.035 73.4205.699马鲛ND1.2150.045 1ND 1.2602.100鳓鱼0.035 44.3690.055 20.026 94.4877.478黄鲫0.0955.2820.1650.1235.6659.442∑HCHs0.15526.1070.3410.205
注:“ND”表示未检测。下同。
2.2.2 滴滴涕
DDTs在采集鱼类中的检出率为100%,且浓度高于HCHs,可能是DDTs的疏水性更强,且生物富集系数更高的原因。龙头鱼、带鱼和鳓鱼的DDTs含量最高,可能是因为龙头鱼的脂肪含量高,DDTs更容易富集在脂肪组织,而带鱼和鳓鱼则可能因为个体较大,生长年份较长,富集DDTs的时间更长所致。其平均浓度依次为DDD>DDE>DDT。DDT使用后,大部分DDT在有氧和厌氧条件下分别缓慢转化为DDE和DDD[33-34],因此,DDT和其代谢物(DDD+DDE)的比值常用来判断DDT的使用时间和生物转化的指标[35],也可以用于分析水环境中DDT的污染来源[29-30,36], DDT/(DDD+DDE)>1表示近期使用过DDT,反之则表示污染来自于早期使用的DDT[29-30,37]。从表4可以看出,所有鱼类样品中DDT/(DDD+DDE)均小于1,表明该水域中近期没有受到新的DDT污染。另外,DDD与DDE的比值能反应DDT的降解方式,DDD/DDE<1表示有氧降解,DDD/DDE>1表示无氧降解[38]。本研究中所有样品中的DDD/DDE比值均为0.9~1.7,说明该水域鱼类DDTs的降解途径大部分是厌氧的。工业上O’P’-DDT与P’P’-DDT的比值在0.2~0.3,试验中O’P’-DDT /P’P’-DDT的比值为0.7~1.4之间,说明该水域最近可能有三氯杀螨醇输入。各鱼类DDTs的EDI值均远小于国标规定的10 μg/kg BW,说明该水域鱼类的DDTs残留量是安全的,但是应该警惕三氯杀螨醇输入后引起的DDTs污染增加。
表4 鱼类滴滴涕农药含量
Table 4 Levels of DDTs in fish
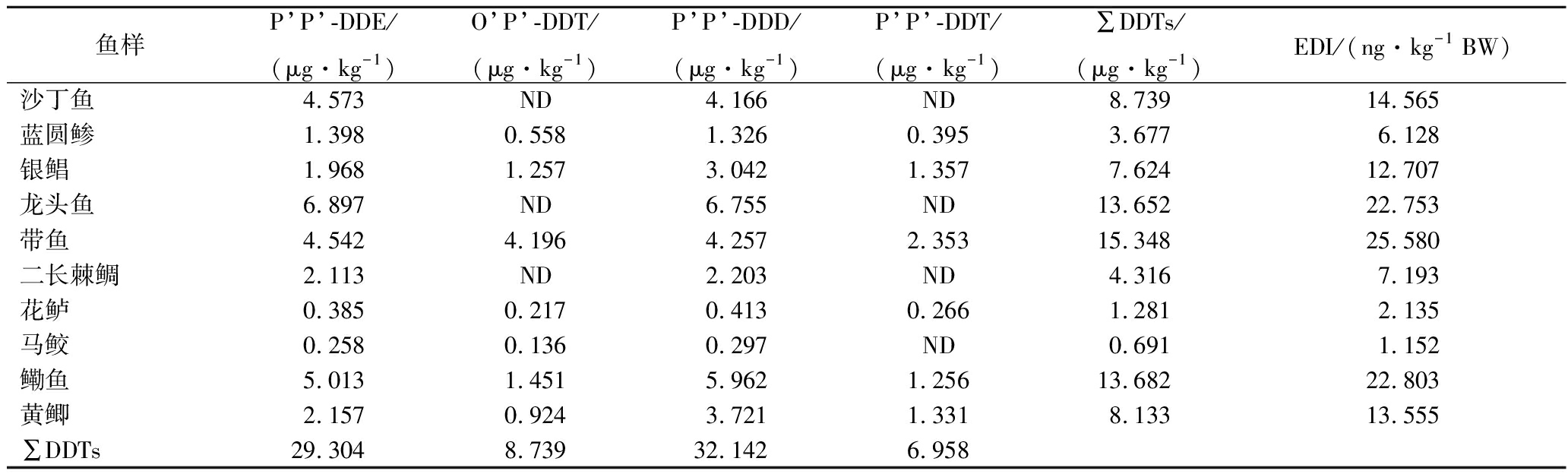
鱼样P’P’-DDE/(μg·kg-1)O’P’-DDT/(μg·kg-1)P’P’-DDD/(μg·kg-1)P’P’-DDT/(μg·kg-1)∑DDTs/(μg·kg-1)EDI/(ng·kg-1 BW)沙丁鱼4.573ND4.166ND8.73914.565蓝圆鲹1.3980.5581.3260.3953.6776.128银鲳1.9681.2573.0421.3577.62412.707龙头鱼6.897ND6.755ND13.65222.753带鱼4.5424.1964.2572.35315.34825.580二长棘鲷2.113ND2.203ND4.3167.193花鲈0.3850.2170.4130.2661.2812.135马鲛0.2580.1360.297ND0.6911.152鳓鱼5.0131.4515.9621.25613.68222.803黄鲫2.1570.9243.7211.3318.13313.555∑DDTs29.3048.73932.1426.958
2.2.3 硫丹、艾氏剂、狄氏剂和异狄氏剂
硫丹由硫丹Ⅰ和硫丹Ⅱ组成,这2种异构体都对光降解具有很强的抵抗力,但是其代谢物硫丹硫酸盐易受光分解的影响[39]。从表5可以看出,所有的鱼肉中都检出硫丹Ⅰ,只有82%的样品检出硫丹Ⅱ,但是抽样鱼类硫丹的平均含量:硫丹Ⅱ>硫丹Ⅰ,这可能是因为硫丹Ⅰ在沉积物中降解更快[40]。工业硫丹产品中硫丹Ⅰ和硫丹Ⅱ的比例约为2.33,可以用两者的比值来判断水域的硫丹污染时间,本研究同时检出2种硫丹的鱼类中硫丹Ⅰ/硫丹Ⅱ均小于1,说明该水域近几年没有受到新的硫丹污染[29-30]。
抽样鱼类中七氯和七氯环氧的检出率分别为80%和90%,其浓度与鱼肉中脂肪含量存在一定的相关性,脂肪含量较高的鱼类中七氯含量较高,与王翀[2]的研究结果一致。从表5可知,七氯和环氧七氯检出浓度都不高,这与汤清清等[41]的研究结果相比有所降低。
艾氏剂和狄氏剂均为环戊二烯结构的杀虫剂,被列入世界卫生组织国际癌症研究机构公布的致癌物清单。两者在所有抽样鱼类中均未检出,因为这类物质在水中的溶解度低,且易于土壤紧密结合,在土壤和水中会缓慢向下迁移。此外,这些极性物质对动物脂肪具有高度的亲和力,容易导致其在食物链的富集。但是,此类物质具有挥发性,能在大气中光降解。这可能会减少水环境中的污染程度[40]。
七氯(七氯和环氧七氯之和)和硫丹(硫丹Ⅰ与硫丹Ⅱ之和)的ADI分别为0.1 μg/kg BW和 6 μg/kg BW,均超过其EDI的100倍以上,说明食用这些鱼类风险很小。
表5 鱼类有机氯农药含量 单位:μg/kg
Table 5 Levels of OCPs in fish

鱼样七氯艾氏剂七氯环氧硫丹Ⅰ硫丹Ⅱ狄氏剂∑七氯∑硫丹沙丁鱼0.074ND0.0340.124NDND0.1080.124蓝圆鲹NDND0.0580.065NDND0.0580.065银鲳0.137ND0.0330.0360.094ND0.170.13龙头鱼0.288ND0.1540.0470.251ND0.4420.298带鱼0.357ND0.0970.1960.417ND0.4540.613二长棘鲷0.158ND0.0320.151NDND0.190.151花鲈NDNDND0.5311.212NDND1.743马鲛0.142ND0.0520.311NDND0.1940.311鳓鱼0.237ND0.0950.4141.031ND0.3321.445黄鲫0.136ND0.0310.3310.417ND0.1670.748∑OCPs1.529ND0.5862.2063.422ND
3 结论
鱼肉样品经QuEChERS提取和净化处理后,用气相色谱检测,质谱确证,方法回收率为83.2%~103.5%,相对标准偏差在4.8%~12.1%,检出限0.01~0.09 μg/kg,实际样品分析结果表明:该法简单、易操作,测定结果准确,能满足鱼肉中多种OCPs的分析要求。采集的10种鱼类均有OCPs检出,含量在5.532~22.174 μg/kg之间,带鱼和鳓鱼的OCPs含量最高,分别为22.174和19.910 μg/kg,P’P’-DDD、P’P’-DDE和β-HCH是最主要的OCPs污染物。各异构体含量的比值表明厦门水域HCHs和DDTs大部分来自早期农药使用残留,但有疑似排入三氯杀螨醇。整体OCPs较往年有所降低[2,41],计算估计每日摄入量均远低于GB2763—2016规定的每日允许摄入量,食用该水域鱼类产生有机氯农药危害的风险较低。
[1] 张家泉, 祁士华,邢新丽,等. 闽江干流沿岸土壤及河口沉积柱中有机氯农药分布特征[J]. 环境科学, 2011, 32(3):673-679.
[2] 王翀. 宁波、舟山群岛经济海产品中有机氯农药的残留、来源及生态风险研究[D]. 杭州:浙江工业大学, 2012.
[3] 徐彪, 孙丙华,姜珊, 等. 巢湖5种鱼类肌肉部位有机氯农药分布特征研究及风险评估[J]. 生物学杂志, 2016, 33(5):19-22.
[4] 丘耀文. 大亚湾海域典型有机氯农药生物累积特征及变化因素研究[J]. 海洋学报, 2007, 29(2):51-58.
[5] JIANG Q T, LEE T K M, CHEN K, et al. Human health risk assessment of organochlorines associated with fish consumption in a coastal city in China[J]. Environmental Pollution, 2005, 136(1):0-165.
[6] 张春辉, 吴永贵,杨少博,等. 广东沿海3种食用鱼中有机氯农药的残留特征及风险评价[J]. 贵州农业科学, 2015, 43(11):174-178.
[7] PANDIT G G, SAHU S K, SHARMA S, et al. Distribution and fate of persistent organochlorine pesticides in coastal marine environment of Mumbai[J]. Environment International, 2006, 32(2):240-243.
[8] 张晓岚, 顾越,李晓静,等. 淀山湖表层水、沉积物和鱼体中有机氯农药的时空变化及人体暴露风险[J]. 上海大学学报(自然科学版), 2016, 22(2):122-130.
[9] 曹方方, 于建钊,费金岩,等. 气相色谱-双柱双电子捕获检测器测定鱼肉中的有机氯农药[J]. 理化检验(化学分册), 2017, 53(3):258-262.
[10] NORLI H R, CHRISTIANSEN A, DERIBE E. Application of QuEChERS method for extraction of selected persistent organic pollutants in fish tissue and analysis by gas chromatography mass spectrometry.[J]. Journal of Chromatography A, 2011, 1218(41):7 234-7 241.
[11] ELHAM J, FATEMEH A, MANSOUR F. Evaluation of Quechers sample preparation and GC mass spectrometry method for the determination of 15 pesticide residues in tomatoes used in salad production plants:[J]. Iranian Journal of Public Health, 2016, 45(2):230-238.
[12] TIRYAKI O. Validation of QuEChERS method for the determination of some pesticide residues in two apple varieties[J]. Journal of Environmental Science & Health,part.b, Pesticides Food Contaminants & Agricultural Wastes, 2016, 51(10):722-729.
[13] ISLAM A K M M, HONG S M, LEE H S, et al. Identification and characterization of matrix components in spinach during QuEChERS sample preparation for pesticide residue analysis by LC-ESI-MS/MS, GC-MS and UPLC-DAD[J]. Journal of Food Science & Technology, 2018,55(10):3 930-3 938.
[14] BILEHAL D C, CHETTI M B, DEEPA G T, et al. Multiresidue pesticide analysis using QuEChERS method in vegetable samples by ultra-performance liquid chromatography[J]. Analytical Chemistry Letters, 2016, 6(6):688-696.
[15] BORDIN A B, MINETTO L, FILHO I D N, et al. Determination of pesticide residues in whole wheat flour using modified QuEChERS and LC-MS/MS[J]. Food Analytical Methods, 2016, 10(1):1-9.
[16] CHO J, LEE J, LIM C U, et al. Quantification of pesticides in food crops using QuEChERS approaches and GC-MS/MS[J]. Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 2016, 33(12):1 803-1 816.
[17] STEINIGER D, LU G, BUTLER J, et al. Determination of multiresidue pesticides in green tea by using a modified QuEChERS extraction and ion-trap gas chromatography/mass spectrometry[J]. Journal of Aoac International, 2010, 93(4):1 169-1 179.
[18] WU C C. Multiresidue method for the determination of pesticides in Oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry[J]. Food Chemistry, 2017, 229:580-587.
[19] HE Z, WANG Y, WANG L, et al. Determination of 255 pesticides in edible vegetable oils using QuEChERS method and gas chromatography tandem mass spectrometry[J]. Analytical and Bioanalytical Chemistry, 2016, 409(4):1 017-1 030.
[20] STREMEL T R D O, DOMINGUES C E, ZITTEL R, et al. Development, validation and matrix effect of a QuEChERS method for the analysis of organochlorine pesticides in fish tissue[J]. Journal of Environmental Science and Health, Part B, 2018:53(4):246-254.
[21] WATANABE K H, DESIMONE F W, THIYAGARAJAH A, et al. Fish tissue quality in the lower Mississippi River and health risks from fish consumption[J]. Science of the Total Environment, 2003, 302(1):109-126.
[22] NICKLAS PAXÉUS. Organic pollutants in the effluents of large wastewater treatment plants in Sweden[J]. Water Research, 1996, 30(5):0-1 122.
[23] BRUGGEMAN W A. Reversed-phase thin-layer chromatography of polynuclear aromatic hydrocarbons and chlorinated biphenyls relationship with hydrophobicity as measured by aqueous solubility and octanol-water partition coefficient[J]. Journal of Chromatography A, 1982, 238(2):335-346.
[24] QIU X, ZHU T, LI J, et al. Organochlorine pesticides in the air around the Taihu Lake, China[J]. Environ.Sci.Technol, 2004, 38(5):1 368-1 374.
[25] ZHOU R, ZHU L, YANG K, et al. Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China[J]. Journal of Hazardous Materials, 2006, 137(1):68-75.
[26] MACKAY D. Correlation of bioconcentration factors[J]. Environmental Science & Technology, 1982, 16(5):274-278.
[27] WALKER K, VALLERO D A, LEWIS R G. Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment[J]. Environmental Science & Technology, 1999, 33(24):4 373-4 378.
[28] KALBITZ, POPP, GEYER, et al. β-HCH mobilization in polluted wetland soils as influenced by dissolved organic matter[J]. Science of the Total Environment, 1997, 204(1):37-48.
[29] UNYIMADU J P, OSIBANJO O, BABAYEMI J O. Polychlorinated biphenyls (PCBs) in River Niger, Nigeria: Occurrence, distribution and composition profiles[J]. Toxicology & Industrial Health, 2017, 34(1):54-67.
[30] UNYIMADU J P, OSIBANJO O, BABAYEMI J O. Selected persistent organic pollutants (POPs) in water of River Niger: occurrence and distribution[J]. Environmental Monitoring & Assessment, 2017, 190(1):6.
[31] IWATA H, TANABE S, SAKAI N, et al. Distribution of persistent organochlorines in the oceanic air and surface seawater and the role of ocean on their global transport and fate[J]. Environmental Science & Technology, 1993, 27(6):495-499.
[32] WILLETT K L, ULRICH E M, HITES R A. Differential toxicity and environmental fates of hexachlorocyclohexane isomers[J]. Environmental Science & Technology, 1998, 32(15):2 197-2 207.
[33] AISLABIE J M, RICHARDS N K, BOUL H L. Microbial degradation of DDT and its residues—A review[J]. New Zealand Journal of Agricultural Research, 1997, 40(2):14.
[34] BAXTER R M. Reductive dechlorination of certain chlorinated organic compounds by reduced hematin compared with their behaviour in the environment[J]. Chemosphere, 1990, 21(4):451-458.
[35] QIAN Y, ZHENG M, ZHANG B, et al. Determination and assessment of HCHs and DDTs residues in sediments from Lake Dongting, China[J]. Environmental Monitoring & Assessment, 2006, 116(1-3):157-167.
[36] GUO Y, YU H Y, ZENG E Y. Occurrence, source diagnosis, and biological effect assessment of DDT and its metabolites in various environmental compartments of the Pearl River Delta, South China: a review.[J]. Environmental Pollution, 2009, 157(6):1 753-1 763.
[37] MA X, YONG R, JIAN G, et al. Concentrations and inventories of polycyclic aromatic hydrocarbons and organochlorine pesticides in watershed soils in the Pearl River Delta, China[J]. Environmental Monitoring & Assessment, 2008, 145(1-3):453-464.
[38] DOONG R A, SUN Y C, LIAO P L, et al. Distribution and fate of organochlorine pesticide residues in sediments from the selected rivers in Taiwan[J]. Chemosphere, 2002, 48(2):237-246.
[39] SCHUPHAN I, SAJKO B, BALLSCHMITER K. The chemical and photochemical degradation of the cyclodien-insecticides aldrin, dieldrin, endosulfan and other hexachloronorbornene derivatives[J]. Zeitschrift Für Naturforschung B, 1972, 27(2):147-156..
[40] UNYIMADU J P, OSIBANJO O, BABAYEMI J O. Levels of organochlorine pesticides in brackish water fish from Niger River, Nigeria[J]. Journal of Environmental & Public Health, 2018, 2018(8):1-9.
[41] 汤清清. 泉州湾鱼体内典型持久性有机污染物水平与食用安全性研究[D]. 厦门:集美大学, 2014.
 -HCH were the main pollutants. The contents and composition of the isomers indicated that most of the OCPs in Xiamen area were come from early pesticide residues, but there was a suspected contamination of DDT caused by the degradation of dicofol. However, the EDI was far lower than daily food intake from the national standard. Therefore, QuEChERS can be applied to the detection of OCPs in marine fish, and the water environment in Xiamen sea area has been less polluted by OCPs recently, indicating low risk of OCPs by fish consumption.
-HCH were the main pollutants. The contents and composition of the isomers indicated that most of the OCPs in Xiamen area were come from early pesticide residues, but there was a suspected contamination of DDT caused by the degradation of dicofol. However, the EDI was far lower than daily food intake from the national standard. Therefore, QuEChERS can be applied to the detection of OCPs in marine fish, and the water environment in Xiamen sea area has been less polluted by OCPs recently, indicating low risk of OCPs by fish consumption.