乳品是人们日常生活中经常食用的食品,含有丰富的营养素。乳中的大量营养素包括蛋白质、乳糖、脂肪,微量营养素包括维生素和矿物质。乳中还含有促进人体健康的生物活性物质,如表皮生长因子、血管内皮生长因子、促红细胞生成素、神经生长因子、生长调节因子、环磷酸腺苷和免疫相关因子如免疫细胞、细胞因子、趋化因子等[1-2]。母乳是新生儿的首要营养物质,其含有丰富且均衡的营养物质,与婴儿的生长发育密切相关。母乳可以通过免疫细胞和免疫分子,例如免疫球蛋白直接抵御病原体[3-5];许多与免疫相关的物质存在于母乳中,它们对婴儿的影响已被广泛认可[6-7]。除了这些免疫成分外,母乳还含有一些非特异性因子,如溶菌酶(通过破坏细菌细胞壁抑制许多细菌的生长)、乳铁蛋白(母乳中的一种多功能蛋白质,它通过刺激细胞因子的产生来限制细菌的生长)和低聚糖(阻止微生物附着于婴儿的黏膜,从而预防感染),可以增强婴儿的免疫功能[8]。
母乳及各种动物乳中还含有一些其他活性成分,可能对机体的健康有益,近期许多研究报道了母乳[9]、牛乳[10]、猪乳[11]和羊乳[12]中miRNA的表达,发现大量的乳中miRNA与免疫调节有关。ZHOU等[13]研究发现,超过60%的免疫相关前体miRNA(pre-miRNA)存在并富集于母乳中。通过对母乳源性miRNA的表达谱研究表明,这些miRNA可以促进婴儿的免疫系统发育,并可能对儿童健康产生有益影响[14-15]。此外,牛乳miRNA可能在抗变态反应和自身免疫性疾病的发展中发挥重要作用[16-17]。本文对miRNA的合成和免疫功能以及miRNA在乳中分离提取和稳定性进行简要概述,并分析了乳中miRNA作为潜在标记物用于检测乳品质量,从而为乳中miRNA的研究提供新方向。
1 miRNA的合成及功能简介
miRNA是真核生物中广泛存在的一种长度大约为22 nt的非编码RNA。miRNA通过调控基因表达进而调控细胞的分化、繁殖、死亡等生理过程[18-20]。miRNA失调在疾病发展中发挥着重要作用,如癌症和糖尿病[21-23]。miRNA主要通过结合靶基因的3’非编码区诱导目标靶mRNA降解或阻断蛋白质翻译,是细胞转录水平的一种重要调控手段。具体合成miRNA的过程为:第一步,miRNA对应基因通过RNA聚合酶Ⅱ转录出初级miRNA(pri-miRNA),pri-miRNA通常包含几百个碱基对;第二步,微加工复合体切割pri-miRNA,同时在Drosha酶和Pasha酶的作用下加工形成约70 nt的“颈环前体”,即pre-miRNA;第三步,在细胞核转运蛋白Exportin5和Ran-GTP等作用下,细胞核中的pre-miRNA被转运到细胞质中,它们在细胞质中经过Dicer酶的剪切和双链RNA结合酶的作用进一步加工成约22 nt的miRNA双链。解链后,成熟的miRNA与RNA的诱导沉默复合体(RNA-induced silencing complex,RISC)结合,然后与目标mRNA互补结合,发挥miRNA的生物学功能,另一条序列则会立即被降解[24]。虽然miRNA研究时间较短,但已成为基因表达调控的热点领域。
2 乳中miRNA的分离提取与稳定性研究
在血浆[25]、血清[25]、唾液[26]、尿液[27]和牛乳[28]中都报道发现了miRNA。乳中miRNA鉴定是乳品研究中的新兴领域,对于乳质量检测和分析有着重要意义。目前一些研究集中在miRNA提供营养方面,miRNA参与很多生物过程,因此人们对miRNA作为牛乳生物活性成分的研究产生了兴趣[29-32]。最近的研究发现包裹在外泌体和微泡中的miRNA可以作为细胞间信使,并在进入其他细胞时影响细胞进程[33-35]。
2.1 乳中miRNA的分离提取
miRNA可以从乳清(whey)、微泡(microvesicle)、外泌体(exosome)中分离提取出来,提取方法见表1。CHEN等[36]用有机法提取了初乳中miRNA;KOSAKA等[9]和IZUMI等[37]用离心柱法提取乳清中miRNA;SUN等[38]和HATA等[10]分别用超速离心法和有机法提取乳中微泡的miRNA;IZUMI等[39]使用有机法提取乳中外泌体的miRNA。HATA等[10]在牛初乳中提取的总RNA含量大约是成熟乳的1.7倍。
2.2 乳中miRNA的稳定性研究
由于体液中同时存在RNA和RNase,miRNA的稳定存在表明它们可以不被RNase消化[25]。将人工合成的RNA(包括miRNA和mRNA)加入牛乳中很快就会被降解,而牛乳本身的miRNA和mRNA却可以在酸性条件及RNA酶处理后仍然保持稳定[36]。母乳中miRNA经RNase、反复冻融、低pH处理没有发生明显的变化,这表明miRNA可以抵抗婴儿胃肠环境,进而被小肠吸收,最终影响婴儿的免疫系统[9]。母乳中存在外泌体和微泡,它们可以将miR-NA包裹在其膜内从而不被降解[33,40]。外泌体和微泡具有磷脂双分子层膜结构,通常由多种细胞分泌而来,其中包裹蛋白质、RNA、miRNA等多种物质,由于磷脂双分子膜结构的存在从而保护miRNA不被降解。因此,由于miRNA特殊的稳定性保证其在母乳中顺利通过婴儿胃肠道被小肠吸收。外泌体对miRNA在肠内的转移具有重要作用,可以防止细胞降解并促进内吞作用[41-42]。CHEN等[36]发现miRNA不仅存在于牛乳中,在液态乳制品、乳粉中也存在miRNA,说明这些miRNA稳定存在于牛乳及牛乳制品中。牛、羊乳被广泛应用于乳品行业,而大多数的婴儿乳粉以牛乳蛋白为基础,有研究显示,在商业巴氏杀菌乳制品中的miRNA可被成年人吸收[43]。此外,有研究表明牛乳外泌体包裹的miRNA可以进入结肠癌细胞[41]、肠细胞[41]、肾细胞[43]、巨噬细胞[44]、以及人外周血单核细胞[43]。
表1 不同来源乳中miRNA分离提取及分析方法总结
Table 1 A summary of miRNAs isolation and analysis methods from different sources of milk
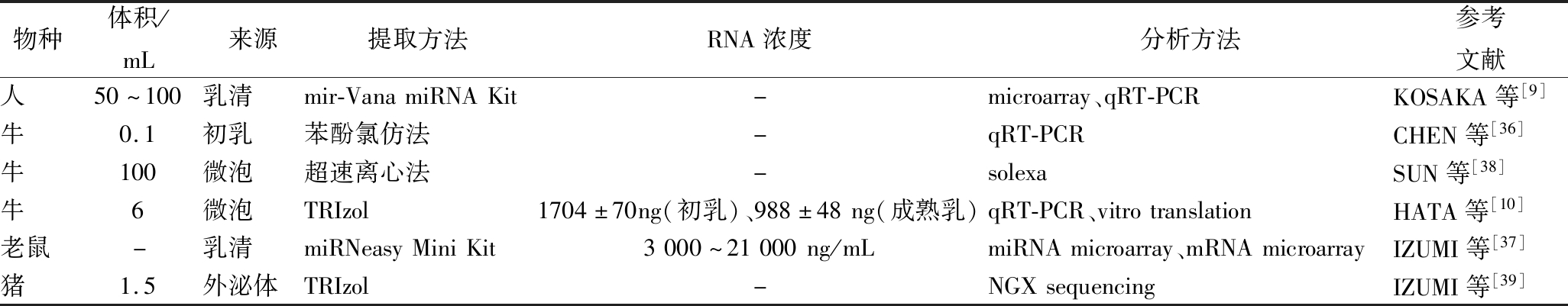
物种体积/mL来源提取方法RNA浓度分析方法参考文献人50~100乳清mir-VanamiRNAKit-microarray、qRT-PCRKOSAKA等[9]牛0.1初乳苯酚氯仿法-qRT-PCRCHEN等[36]牛100微泡超速离心法-solexaSUN等[38]牛6微泡TRIzol1704±70ng(初乳)、988±48ng(成熟乳)qRT-PCR、vitrotranslationHATA等[10]老鼠-乳清miRNeasyMiniKit3000~21000ng/mLmiRNAmicroarray、mRNAmicroarrayIZUMI等[37]猪1.5外泌体TRIzol-NGXsequencingIZUMI等[39]
3 乳中miRNA的免疫功能研究
研究发现,miRNA在调控重要生物过程的基因表达中发挥作用,包括细胞增殖和分化、组织发育和免疫反应[5,9,29]。miRNA有助于调节免疫细胞的发育,调节先天免疫反应和获得性免疫反应[5]。最近有研究表明,细胞外的miRNA通过包裹在外泌体或微泡中,稳定地存在于体液中,可能在免疫应答过程或在细胞间信息交流中扮演重要角色[9,27,45]。许多miRNA参与各种免疫相关的信号传导网络,miRNA可以调控免疫细胞的各个方面,如发育、增殖、成熟、存活、稳态、分化、转移、细胞因子表达[45]。例如在T细胞中,miR-181a调节细胞敏感性和增殖;miR-155调节CD8+T细胞应答;miR-146a通过NF-kB信号激活T细胞受体。
在不同的泌乳阶段,乳中miRNA表达量不同。与成熟乳相比,牛初乳中的miRNA具有更高的表达[36,38,44]。初乳的外泌体和微泡中所含免疫相关的miRNA含量高于成熟乳,并且这些miRNA可以进入受体细胞中影响基因表达;初乳中富含抗体、溶菌酶、白细胞、寡糖等成分都具有免疫功能[38]。可见,泌乳早期的乳中含有更高含量的miRNA,可以在提供免疫保护方面发挥作用。
3.1 母乳miRNA免疫功能
2010年KOSAKA等[9]首次发现母乳中含有miRNA,且哺乳期前6个月乳中与免疫相关的miRNA含量较高,随后其含量随着时间的推移而逐步下降。与其他体液相比,母乳中含有大量的总RNA,母乳中高含量miRNA已被认为与免疫相关[9,27,45]。免疫相关的miR-155、miR-181a、miR-181b、miR-17-92、miR-125b、miR-146b、miR-223 和let-7i,在母乳乳清中广泛存在[9];免疫相关的miR-148a-3p、miR-30b-5p、miR-182-5p、miR-200a-3p、miR-29a-3p、miR-141-3p、miR-146b-5p、let-7f-1-5p、let-7f-2-5p、let-7a-2-5p和let-7a-3-5p在母乳外泌体中广泛存在[13];miRNA不仅在母乳的乳清和外泌体中存在,也在母乳的脂质中广泛存在,与免疫相关的miRNA有hsa-miR-148a、hsa-let-7a、hsa-miR-200c、hsa-miR-146b-5p、hsa-let-7f、hsa-miR-30d、hsa-let-7b和hsa-miR-21[46]。
3.2 牛乳miRNA免疫功能
牛乳一直以来都是人类广泛食用的乳制品,也用于制造婴儿配方乳粉。在牛乳中发现的miRNA其中大部分是与免疫相关的。IZUMI等[44]在牛乳清中发现与免疫相关的miRNA,包括miR-15b、miR-27b、miR-106b、miR-155、miR-223、miR-34a、miR-130a等。在牛乳微泡中发现与免疫相关的miRNA,包括miR-24、miR-30d、miR-93、miR-106a、miR-181a、miR-200a、miR-451、miR-223等[47-53]。
3.3 猪乳miRNA免疫功能
GU等[11]在猪乳外泌体中发现了12种猪特异的且含量丰富的miRNA,分别是miR-148a-3p、miR-182-5p、miR-200c-3p、miR-30d-5p、miR-574-3p、let-7a-1-5p、miR-30c-2-5p、miR-30c-1-5p、miR-191-5p、miR-375-3p、miR-27b-3p和miR-21-5p,这些miRNA被证明具有免疫功能。
4 乳中miRNA作为质量控制指标的分析
近年来,乳制品安全事件频繁出现,许多不法商贩通过向牛乳中掺水牟取不正当利益。由于向牛乳中加水会导致除水以外的各项指标降低,因此,为了提高各项检测指标,不法商贩在牛乳中添加水后又向其中加入各种添加剂,并通过反复勾兑来使牛乳达标。最常用的添加剂有以下几种:蛋白(提高蛋白指数)、乳清粉(提高各项指数)、糊精、脂肪油(提高脂肪指数)、二合一(可提高相应各项指数,对蛋白指数最有效)[54]。其中,“二合一”的典型案例是三鹿乳粉事件中不法商贩向牛乳中加水后又加入三聚氰胺。
现有的检测牛乳质量的方法主要是检测蛋白质、脂肪和碳水化合物等标志物。虽然这些标志物易于制定行业标准,但都容易被人为操纵,因而难以实现牛乳质量的检测。最根本的牛乳质量检测方法是应找到一种在牛乳中稳定存在,而稀释后该成分会变化,但不受添加剂影响的物质。DNA分子以其高稳定性逐渐成为食品品种鉴定的目标分析物,基于DNA运用PCR技术检测不同动物乳的掺假[55-56]。早在2014年,刘永峰等[57]建立了从牛乳中提取大片段DNA(>1000 bp)的方法,并在后续的研究中优化和扩展了DNA的提取方法[58-60],基于这些方法提取乳粉中的DNA用于检测乳粉掺假,通过PCR扩增,最低检测到牛乳粉中掺假0.1%的羊乳粉[61]。DNA可以用于乳品质量安全研究,同样作为核酸的RNA也有相应研究结果。张辰宇等[54]发现miRNA存在于原乳、商品液态乳制品及乳粉中,牛乳中的特异性miRNA图谱不受任何添加剂的影响,并且与其他动物中miRNA图谱不同。牛乳中特异性miRNA图谱是一种用于检测牛乳质量的完美标志物。因此,通过检测这些miRNA中的任意一种或几种就可以确定原乳的质量,从而杜绝不法分子向牛乳中掺入其他物质的可能。
CHEN等[36]研究表明,通过检测乳制品中的蛋白质或氮含量,已经不能真实地反映乳品质,但通过检测特定的miRNA含量能准确地反映乳品质,评估正常乳和掺假乳之间的miRNA水平,比检测蛋白质或氮含量更可靠[36]。因此,评估正常牛乳和掺假牛乳的miRNA水平比其他标记物,如蛋白质、氮等,提供了更好的牛乳质量控制选择。
张辰宇等[62]通过检测乳制品中的miRNA进而鉴别乳制品,研究发现,若所测得的miRNA种类及含量与初乳样品的miRNA表达谱相近或相同,则鉴定此样品为初乳制品;若所测得的miRNA种类及其含量与成熟乳样品的miRNA表达谱相近或相同,则鉴定为此样品为成熟乳制品。因此,牛乳中所含miRNA可以作为潜在的牛乳质量标记物,将很有可能给整个乳制品行业,尤其是牛乳质量控制领域带来一场革命。
5 展望
在相关科学研究中,应结合实际情况选择最优的乳中miRNA提取方法。乳中外泌体和微泡中包裹miRNA在细胞中发挥重要作用,但具体机制还有待于深入探索。母乳中miRNA具有免疫功能,通过哺乳传递给婴儿,现有的婴幼儿配方乳粉中未考虑将miRNA作为营养素进行添加,期望通过未来对乳中miRNA的深入研究可以阐明各种miRNA对机体的作用机制,从而有利于研制出适用于不同婴幼儿的含miRNA的配方乳粉或辅助食品。
[1] BALLARDO,MORROWA L.Human milk composition:nutrients and bioactive factors[J].Pediatric Clinics of North America,2013,60(1):49-74.
[2] 刘永峰,赵晓微,刘满顺,等.牛奶乳清液对小鼠学习记忆能力的影响[J].陕西师范大学学报(自然科学版),2016,44(4):108-113;124.
[3] BARTE L D P.MicroRNAs:genomics,biogenesis,mechanism,and function[J].Cell,2004,116(2):281-297.
[4] LEWIS B P,SHIH I H,JONES-RHOADES M W,et al.Prediction of mammalian microRNA targets[J].Cell,2003,115(7):787-798.
[5] XIAO C,RAJEWSKY K.MicroRNA control in the immune system:basic principles[J].Cell,2009,136(1):26-36.
[6] GOLDMAN A S.The immune system in human milk and the developing infant[J].Breastfeeding Medicine,2007,2(4):195-204.
[7] NEWBURG D S,WALKER W A.Protection of the neonate by the innate immune system of developing gut and of human milk[J].Pediatric Research,2007,61(1):2-8.
[8] CARVER J D,PIMENTEL B,COX W I,et al.Dietary nucleotide effects upon immune function in infants[J].Pediatric Clinics of North America,1991,88(2):359-363.
[9] KOSAKA N,IZUMI H,SEKINE K,et al.MicroRNA as a new immune-regulatory agent in breast milk[J].Silence,2010,1(1):7.
[10] HATA T,MURAKAMI K,NAKATANI H,et al.Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs[J].Biochemical and Biophysical Research Communications,2010,396(2):528-533.
[11] GU Y,LI M,WANG T,et al.Lactation-related microRNA expression profiles of porcine breast milk exosomes[J].PLoS One,2012,7(8):e43691.
[12] NA R S,E G X,SUN W,et al.Expressional analysis of immune-related miRNAs in breast milk[J].Genetics and Molecular Research:GMR,2015,14(3):11 371-11 376.
[13] ZHOU Q,LI M,WANG X,et al.Immune-related microRNAs are abundant in breast milk exosomes[J].International Journal of Biological Sciences,2012,8(1):118-123.
[14] ADMYRE C,JOHANSSON S M,QAZI K R,et al.Exosomes with immune modulatory features are present in human breast milk[J].Journal of Immunology,2007,179(3):1 969-1 978.
[15] ADMYRE C,TELEMO E,ALMQVIST N,et al.Exosomes-nanovesicles with possible roles in allergic inflammation[J].Allergy,2008,63(4):404-408.
[16] KIRCHNER B,PFAFFL M W,DUMPLER J,et al.MicroRNA in native and processed cow's milk and its implication for the farm milk effect on asthma[J].Journal of Allergy and Clinical Immunology,2016,137(6):1 893-1 895.e13.
[17] MELNIK B C,JOHN S M,CARRERA-BASTOS P,et al.Milk:a postnatal imprinting system stabilizing FoxP3 expression and regulatory T cell differentiation[J].Clinical and Translational Allergy,2016,6(1):18.
[18] AMBROS V.The functions of animal microRNAs[J].Nature,2004,431(7 006):350-355.
[19] DE GUIRE V,CARON M,SCOTT N,et al.Designing small multiple-target artificial RNAs[J].Nucleic Acids Research,2010,38(13):e140.
[20] SYLVESTRE Y,DE GUIRE V,QUERIDO E,et al.An E2F/miR-20a autoregulatory feedback loop[J].Journal of Biological Chemistry,2007,282(4):2 135-2 143.
[21] STAHLHUTESPINOSA C E,SLACK F J.Cancer issue:the role of microRNAs in cancer[J].Yale Journal of Biology and Medicine,2007,79(3-4):131-140.
[22] EIS P S,TAM W,SUN L P,et al.Accumulation of miR-15 5 and BIC RNA in human B cell lymphomas[J].Proceedings of the National Academy of Sciences of the United States of America,2005,102(10):3 627-3 632.
[23] FENG J,XING W,XIE L.Regulatory roles of microRNAs in diabetes[J].International Journal of Molecular Sciences,2016,17(10):1 729.
[24] YANG M,MATTES J.Discovery,biology and therapeutic potential of RNA interference,microRNA and antagomirs[J].Pharmacology &Therapeutics,2008,117(1):94-104.
[25] CHEN X,BA Y,MA L,et al.Characterization of micro RNAs in serum:a novel class of biomarkers for diagnosis of cancer and other diseases[J].Cell Research,2008,18(10):997-1 006.
[26] MICHAEL A,BAJRACHARYA S D,YUEN P S,et al.Exosomes from human saliva as a source of microRNA biomarkers[J].Oral Diseases,2010,16(1):34-38.
[27] WEBER J A,BAXTER D H,ZHANG S,et al.The microRNA spectrum in 12 body fluids[J].Clinical Chemistry,2010,56(11):1 733-1 741.
[28] ZHOU Q,LI M,WANG X,et al.Immune-related microRNAs are abundant in breast milk exosomes[J].International Journal of Biological Sciences,2012,8(1):118-123.
[29] CHEN T,XIE M Y,SUN J J,et al.Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells[J].Scientific Reports,2016,6:33 862.
[30] LONNERDAL B,DU X G,LIAO Y L,et al.Human milk exosomes resist digestion in vitro and are internalized by human intestinal cells[J].Faseb Journal,2015,29 (1 supplement):121.3.
[31] IZUMI H,TSUDA M,SATO Y,et al.Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages[J].Journal of Dairy Science,2015,98(5):2 920-2 933.
[32] ALSAWEED M,LAI C T,HARTMANN P E,et al.Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk [J].Scientific Reports,2016,6(1-3):20 680.
[33] VALADI H,EKSTROM K,BOSSIOS A,et al.Exosome mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J].Nature Cell Biology,2007,9(6):654-659.
[34] SKOG J,WURDINGER T,VAN RIJN S,et al.Glioblastom a microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers[J].Nature Cell Biology,2008,10(12):1 470-1 476.
[35] YUAN A,FARBER E L,RAPOPORT A L,et al.Transfer of microRNAs by embryonic stem cell microvesicles[J].PLoS One,2009,4(3):e4 722.
[36] CHEN X,GAO C,LI H,et al.Identification and characterization of microRNAs in raw milk during different periods of lactation,commercial fluid,and powdered milk products[J].Cell Research,2010,20(10):1 128-1 137.
[37] IZUMI H,KOSAKA N,SHIMIZU T,et al.Time-dependent expression profiles of microRNAs and mRNAs in rat milk whey[J].PLoS One,2014,9(2):e88 843.
[38] SUN Q,CHEN X,YU J,et al.Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum[J].Protein &Cell,2013,4(3):197-210.
[39] CHEN T,XI Q Y,YE R S,et al.Exploration of microRNAs in porcine milk exosomes[J].BMC Genomics,2014,15(1):100.
[40] HUNTER M P,ISMAIL N,ZHANG X L,et al.Detection of microRNA expression in human peripheral blood microvesicles[J].PLoS One,2008,3(11):e3 694.
[41] WOLF T,BAIER S R,ZEMPLENI J.The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma caco-2 cells and rat small intestinal IEC-6 cells[J].Journal of Nutrition,2015,145(10):2 201-2 206.
[42] KUSUMA R J,MANCA S,FRIEMEL T,et al.Human vascular endothelial cells transport foreign exosomes from cow′s milk by endocytosis[J].America Journal of Physiology-Cell Physiology,2016,310(10):C800-C807.
[43] BAIER SR,NGUYEN C,XIE F,et al.MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells,HEK-293 kidney cell cultures,and mouse livers[J].Journal of Nutrition,2014,144(10):1 495-1 500.
[44] IZUMI H,KOSAKA N,SHIMIZU T,et al.Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions[J].Journal of Dairy Science,2012,95(9):4 831-4 841.
[45] CHEN K D,GOTO S,HSU L W,et al.Identification of miR-27b as a novel signature from the mRNA profiles of adipose-derived mesenchymal stem cells involved in the tolerogenic response[J].PLoS One,2013,8(4):e60 492.
[46] MUNCH E M,HARRIS R A,MOHAMMAD M,et al.Transcriptome profiling of microRNA by next-gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk[J].PLoS One,2013,8(2):e50 564.
[47] ROY L,BIKORIMANA E,LAPID D,et al.miR-24 is required for hematopoietic differentiation of mouse embryonic stem cells[J].PLoS Genetics,2015,11(1):e1 004 959.
[48] GAZIEL-SOVRAN A,SEGURA M F,DI MICCO R,et al.miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis[J].Cancer Cell,2011,20(1):104-118.
[49] SHARMA A,MANISH K,AICH J,et al.Post-transcriptional regulation of interleukin-10 expression by hsa-miR-106a[J].Proceedings of the National Academy of Sciences,2009,106(14):5 761-5 766.
[50] LI Q J,CHAU J,SYLVESTER G,et al.miR-181a is an intrinsic modulator of T cell sensitivity and selection[J].Cell,2007,129(1):147-161.
[51] NAVARRO A,GAYA A,MARTINEZ A,et al.miroRNA expression profiling in classic Hodgkin lymphoma[J].Blood,2008,111(5):2 825-2 832.
[52] BANDRES E,BITARTE N,ARIAS F,et al.microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells[J].Clinical Cancer Research,2009,15(7):2 281-2 290.
[53] CHEN Q,WANG H,LIU Y,et al.Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1b production in macrophages by targeting STAT3[J].PLoS One,2012,7(8):e42 971.
[54] 张辰宇,曾科,张俊峰,等.用于牛乳质量检测的标志物、方法、生物芯片和试剂盒:江苏,CN102666878A[P].2012-09-12.
[55] KOTOWICZ M,ADAMCZYK E,BANIA J.Application of a duplex-PCR for detection of cows' milk in goats' milk[J].Annals of Agricultural Environmental Medicine,2008,14(2):215-218.
[56] HUTU I,BOIDURA O,POPESCU S,et al.A multiplex PCR approach to the quantification of cow milk in goat products[J].Current Opinion in Biotechnology,2013,24:S95.
[57] LIU Y F,GAO J L,YANG Y F,et al.Novel extraction method of genomic DNA suitable for long-fragment amplification from small amounts of milk[J].Journal of Dairy Science,2014,97(11):6 804-6 809.
[58] 刘永峰,库婷,高俊岭,等.超低温冻藏牛奶中牛基因组DNA的提取方法[J].陕西师范大学学报(自然科学版),2015,43(6):94-99.
[59] LIAO J,LIU Y F,YANG L,et al.Development of a rapid mitochondrial DNA extraction method for species identification in milk and milk products[J].Journal of Dairy Science,2017,100(9):7 035-7 040.
[60] LIAO J,LIU Y F.Purification procedures meaningfully influence DNA quantification in milk[J].LWT-Food Science and Technology,2018,94:8-12.
[61] LIAO J,LIU Y F,YANG L,et al.Development of a rapid mitochondrial DNA extraction method for species identification in milk and milk products[J].Journal of Dairy Science,2017,100(9):7 035-7 040.
[62] 张辰宇,斯琴,锡林其其格,等.人乳微小核糖核酸及其应用:北京,CN103651811A[P].2014-03-26.