L-丝氨酸是化学和材料领域中最重要的30个骨架化合物之一,亦被广泛运用于医药、化工、食品等多个领域[1-4],也是世界氨基酸行业中工业化生产难度较大的氨基酸。目前,工业生产L-丝氨酸的方法主要有蛋白水解法[5]、化学合成法[6]和酶转化法[7]等,其中蛋白水解法存在工艺复杂、分离精制困难等缺点;化学合成法存在污染重、D-丝氨酸与L-丝氨酸分离困难等缺点;酶转化法是目前国际上普遍采用的方法,但仍存在转化率偏低、前体物质价格昂贵等问题。因此,以微生物利用廉价的糖质原料发酵生产L-丝氨酸正成为研究热点[8]。
目前微生物发酵法生产L-丝氨酸的研究主要集中于大肠杆菌和谷氨酸棒杆菌上。MUNDHADA等[9]将大肠杆菌Q1进行适应性进化(ALE),经补料分批发酵,L-丝氨酸的产量达到37 g/L。谷氨酸棒杆菌(Corynebacterium glutamicum)是发酵生产氨基酸的重要菌株,目前对谷氨酸棒杆菌产L-丝氨酸的研究集中于解除产物反馈抑制、增强合成途径的表达及敲除降解途径等代谢改造,L-丝氨酸在谷氨酸棒杆菌中的代谢涉及合成途径3个关键酶和降解途径2个关键酶,即甘油酸脱氢酶(PGDH,编码基因serA)、磷酸丝氨酸氨基转移酶(PSAT,编码基因serC)和磷酸丝氨酸磷酸化酶(PSP,编码基因serB);丝氨酸脱氢酶(SerDH,编码基因sdaA)及丝氨酸羟甲基转移酶(SHMT,编码基因glyA)。PETERS-WENDISCH等[10-11]以C.glutamicum ATCC 13032作为出发菌株,敲除降解途径的丝氨酸脱水酶编码基因sdaA,解除反馈抑制并表达合成途径关键酶基因serAΔ197、serC和serB,调控L-丝氨酸降解途径关键酶丝氨酸羟甲基转移酶表达水平,构建的重组菌L-丝氨酸的产量为36.26 g/L。本课题组[12]从自然界筛选得到1株能够直接利用糖质原料发酵生产6.65 g/L L-丝氨酸的菌株C. glutamicum SYPS-062,通过传统诱变[13]、解除L-丝氨酸反馈抑制及敲除副产物代谢途径,得到菌株ΔSSAAI,L-丝氨酸产量为26.23 g/L[14],对菌株ΔSSAAI进行等离子诱变,得到突变菌株A36,L-丝氨酸产量达到30.57 g/L[15]。
虽然在代谢工程改造谷氨酸棒杆菌生产L-丝氨酸方面取得了一定进展,但还存在菌株高产机理不清晰,菌株发酵周期过长等问题,如能通过比较基因组分析初步解析谷氨酸棒杆菌高产L-丝氨酸机制,则可为进一步理性改造L-丝氨酸高产菌株提供新的思路。本研究在对2株不同表型的产L-丝氨酸谷氨酸棒杆菌进行全基因组比较分析基础上[15],对其中与转运或代谢相关的4个发生氨基酸突变的基因(folC、faS、rhtA和tyrP)进行研究,通过回复突变、基因敲除与加强表达,阐明这些基因与菌株产L-丝氨酸及生长之间的关系。
1 材料与方法
1.1 材料
1.1.1 菌株和引物
产L-丝氨酸的谷氨酸棒杆菌ΔSSAAI与其突变菌株A36为实验室前期构建并保藏;pK18mobsacB(缩写pK18) 敲除质粒为实验室保藏[16]。大肠杆菌JM109为实验室保藏,大肠杆菌-谷氨酸棒杆菌穿梭表达质粒pDXW-10(缩写pD)为江南大学王小元教授惠赠[17]。大肠杆菌-谷氨酸棒杆菌穿梭诱导表达质粒pZM1(缩写pM)为美国伦斯勒理工学院Mattheos A.G.Koffas教授惠赠(doi.org/10.1186/s12934-018-0990-z),引物详细信息见表1,菌株与质粒信息见表2。所用同源重组酶购自南京诺唯赞生物科技有限公司,胶回收和质粒提取试剂盒购自上海捷瑞生物工程有限公司。限制性内切酶EcoR I,Xba I,Nde I,BamH I和Bgl II购自TaKaRa公司。
1.1.2 培养基
(1)种子固体培养基(g/L):脑心浸液(BHI)37,葡萄糖20,(NH4)2SO4 10,K2HPO4 0.2,NaH2PO40.3,MgSO4·7H2O 0.5,琼脂粉20。
(2)种子液体培养基(g/L):脑心浸液(BHI)37,葡萄糖20,(NH4)2SO4 10,K2HPO4 0.2,NaH2PO40.3,MgSO4·7H2O 0.5;装液量20 mL/250 mL。
表1 本研究所用的引物
Table 1 Primer used in this study

引物序列(5′-3′)目标folC-FCTATGACATGATTACGAATTCACGAGTGCCTCAAACTTGCfolC-RTGCCTGCAGGTCGACTCTAGGCTGATGCAGCTAGGCGCATTGfolC 下游突变引物faS-FCTATGACATGATTACGAATTAAGGCAACCCTGACCCGCTCfaS-RTGCCTGCAGGTCGACTCTAGACAAGTCCACCGATCTGGAAfaS 下游突变引物rhtA-FCTATGACATGATTACGAATTGCATGCTTTTTATGGTAGTTrhtA-RTGCCTGCAGGTCGACTCTAGCTGCGGCTGCGATTATCAAArhtA 下游突变引物tyrP-FCTATGACATGATTACGAATTCCACCAATCGTGGGCACCCTtyrP-RTGCCTGCAGGTCGACTCTAGCCCATTATCACCCATAAAAtyrP 下游突变引物pZM1-r-FAAAAGGAGGACAACCATATGGTGAATGATGCTGGCTTGApZM1-r-RTCTGCAGCGGCCGCGGATCCCTAGGAGGGGCGTCGCAAA质粒 pZM1过表达rhtApDXW-10-r-FTCACACAGGAAACAGAATTCGTGAATGATGCTGGCTTGApDXW-10-r-RCAGCTTAAGCCGCGGAGATCCTAGGAGGGGCGTCGCAAA质粒pDXW-10过表达rhtApZM1-t-FAAAAGGAGGACAACCATATGATGACTACCGAATCAATAGpZM1-t-RTCTGCAGCGGCCGCGGATCCCTATGCCCAACCCGCAGG质粒pZM1过表达tyrPpDXW-10-t-FTCACACAGGAAACAGAATTCATGACTACCGAATCAATAGpDXW-10-t-RCAGCTTAAGCCGCGGAGATCCTATGCCCAACCCGCAGG质粒pDXW-10过表达tyrPpK18-rhtA-1CTATGACATGATTACGAATTTGGTGAGGAAGCTGTTGCATpK18-rhtA -2AGACAGACATGTGGAGACCCACGCCGTTAACCACCATCApK18-rhtA -3GGTCTCCACATGTCTGTCTTCCTAGCGGTTTCCCACACpK18-rhtA-4TGCCTGCAGGTCGACTCTAGCCGAGGAGGGTAAGCCAGTrhtA敲除pK18-tyrP-1CTATGACATGATTACGAATTACGATGATCGCGGCAACCpK18-tyrP-2TCAGAATGTGTCCTCGATCGTCGGATTAATGCCTGpK18-tyrP-3ATTAATCCGACGATCGAGGACACATTCTGAGGTGCpK18-tyrP-4TGCCTGCAGGTCGACTCTAGAGCCATCACCGACGGCGCCCtyrP敲除
注:下划线代表同源臂
表2 菌株与质粒
Table 2 Strains and plasmids

菌株/质粒描述来源pK18mobsacB(pK18)Integration vector, oriV, oriT, mob, sacB,Km实验室保存pDXW-10(pD)E.coli-C.glutamicum shuttle-constitutive expression plasmid实验室保存pZM1(pM)E.coli-C.glutamicum shuttle-induced expression plasmid实验室保存pK18-folCpK18mobsacB connected to folC fragment on A36本研究pK18-faSpK18mobsacB connected to faS fragment on A36本研究pK18-rhtApK18mobsacB connected to rhtA fragment on A36 本研究pK18-tyrPpK18mobsacB connected to tyrP fragment on A36 本研究pK18-RpK18mobsacB connected to partially deleted rhtA gene本研究pK18-TpK18mobsacB connected to partially deleted tyrP gene本研究pZM1-rhtAInducible expression plasmid containing rhtA fragment本研究pZM1-tyrPInducible expression plasmid containing tyrP fragment本研究pDXW-10-rhtAConstitutive plasmid containing rhtA fragment本研究pDXW-10-tyrPConstitutive plasmid containing tyrP fragment本研究KfReverse mutation folC on ΔSSAAI本研究KoReverse mutation faS on ΔSSAAI本研究KrReverse mutation rhtA on ΔSSAAI本研究KtReverse mutation tyrP on ΔSSAAI本研究ΔrhtAKnockout gene rhtA on ΔSSAAI本研究ΔtyrPKnockout gene tyrP on ΔSSAAI本研究pM-ROverexpression of rhtA with inducible plasmid pZM1 on A36本研究pM-TOverexpression of tyrP with inducible plasmid pZM1 on A36本研究pD-ROverexpression of rhtA with a constitutive plasmid pDXW-10 on A36本研究pD-TOverexpression of tyrP with a constitutive plasmid pDXW-10 on A36本研究
(3)发酵培养基(g/L):蔗糖100,(NH4)2SO4 30,KH2PO4 3,MgSO4·7H2O 0.5,FeSO4·7H2O 0.02,MnSO4·H2O 0.02,原儿茶酸(PCA)0.03,生物素5×10-5,VB1 4.5×10-4;装液量25 mL/250 mL,CaCO3 1.5 g/25 mL;
(4)谷氨酸棒杆菌感受态培养基(g/L):蛋白胨 10,酵母粉 5,NaCl 10,Tween-80 1,甘氨酸 25,异烟肼 0.04。
(5)蔗糖筛选培养基(g/L):脑心浸液(BHI)37,蔗糖100,(NH4)2SO4 10,K2HPO4 0.2,NaH2PO40.3,MgSO4·7H2O 0.5,琼脂粉20。
1.2 实验方法
1.2.1 质粒的构建
回复突变质粒构建:以rhtA基因回复突变为例,以菌株A36的基因组为模板,选择点突变前500 bp和后500 bp为扩增目标,利用引物rhtA-F、rhtA-R扩增含点突变的同源臂基因片段。通过单片段同源重组酶将含同源臂的目的片段与经EcoR I和Xba I双酶切的质粒pK18mobsacB连接,构建回复突变质粒。
敲除重组质粒的构建:基因敲除也采用质粒pK18mobsacB,以敲除基因tyrp为例,以ΔSSAAI的基因组为模板,分别利用pK-18-tyrp-1/2、pK-18-tyrp-3/4扩增tyrp的上下游同源臂片段,上下游同源片段与EcoR I和Xba I双酶切的质粒pK18mobsacB连接,得到敲除同源重组质粒。
诱导型加强表达质粒(IPTG诱导)的构建:以加强表达rhtA为例,以A36的基因组为模板,利用引物pZM1-r-F/R 扩增基因rhtA,目的基因rhtA采用单片段同源重组酶与经Nde I和BamH I双酶切的线性化质粒pZM1连接,得到诱导型加强表达重组质粒。
组成型加强表达质粒的构建:以加强表达rhtA为例,以A36的基因组为模板,利用引物pDXW-10-r-F/R 扩增基因rhtA,目的基因rhtA采用单片段同源重组酶与经EcoR I和Bgl II双酶切的线性化质粒pDXW-10连接,得到组成型加强表达重组质粒。
1.2.2 重组菌的构建
回复突变菌株与敲除菌株的构建:制备ΔSSAAI及A36感受态,将10 μL质粒与100 μL感受态细胞混合加入电极杯中,在1.8 kV电压下,5 ms电击2次。将电击的感受态细胞加入恢复液中放置于金属浴(46 ℃)上温育6 min,转置于摇床上(120 r/min,30 ℃)温育2 h,然后将其涂布电转化谷氨酸棒杆菌培养基中(添加硫酸卡那霉素50 mg/L)。挑取阳性转化子扩大培养,稀释一定浓度涂布至100 g/L蔗糖筛选培养基上,进行二次同源重组筛选;挑取转化子进行PCR验证及测序验证。
诱导型加强表达与组成型加强表达菌株的构建:将诱导型加强表达重组质粒或组成型加强表达重组质粒转化感受态细胞,挑取阳性转化子,进行PCR验证及测序验证。
1.2.3 发酵方法
用接种环挑取1环菌落接种至种子液体培养基中(20 mL/250 mL),于30 ℃,120 r/min往复式摇床培养,至对数生长期(生物量OD562=20~25),按照体积分数为4%接种量接种至发酵培养基中(25 mL/250 mL),发酵培养基的初始OD562≈1,于30 ℃,120 r/min往复式摇床培养120 h,每隔12 h取样测定生物量及L-丝氨酸产量。
1.2.4 分析方法
生物量的测定: 采用1 mol/L的HCl稀释发酵液至适当倍数,然后利用紫外分光光度计于562 nm处测定OD562作为菌体量。
L-丝氨酸的测定:利用HPLC测定样品中的L-丝氨酸含量[15]。
2 结果与分析
2.1 基因突变对菌株生长和产L-丝氨酸影响
在ΔSSAAI中利用pK18-faS、pK18-folC、pK18-rhtA、pK18-tyrP分别对faS、folC、rhtA和tyrP四个基因进行回复突变,基因片段大小如图1所示,测序结果显示质粒构建成功。
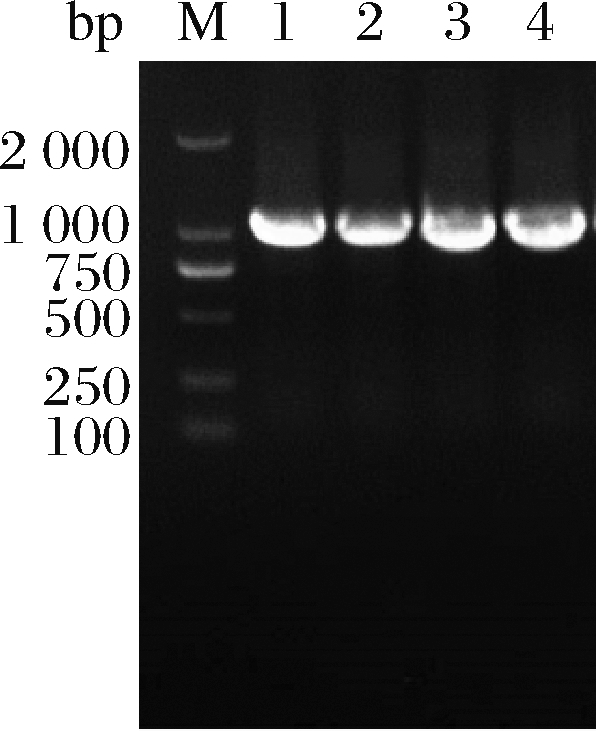
M-Marker;1-rhtA PCR片段;2-tyrP PCR片段;
3-folC PCR片段;4-faS PCR片段
图1 质粒pK18-rhtA、pK18-tyrP、pK18-folC、pK18-faS
电泳验证
Fig.1 Electrophoresis verification of pK18-rhtA, pK18-
tyrP,pK18-folC, pK18-faS
利用上述重组质粒经二次同源重组分别构建回复突变菌株Kf、Ko、Kr和Kt,再对菌株Kf、Ko、Kr和Kt中faS、folC、rhtA、tyrP基因分别进行测序,测序结果正确的表明回复突变菌株构建成功。将回复突变菌株进行发酵,考察基因突变对菌株生长和产L-丝氨酸的影响,发酵结果如图2所示。
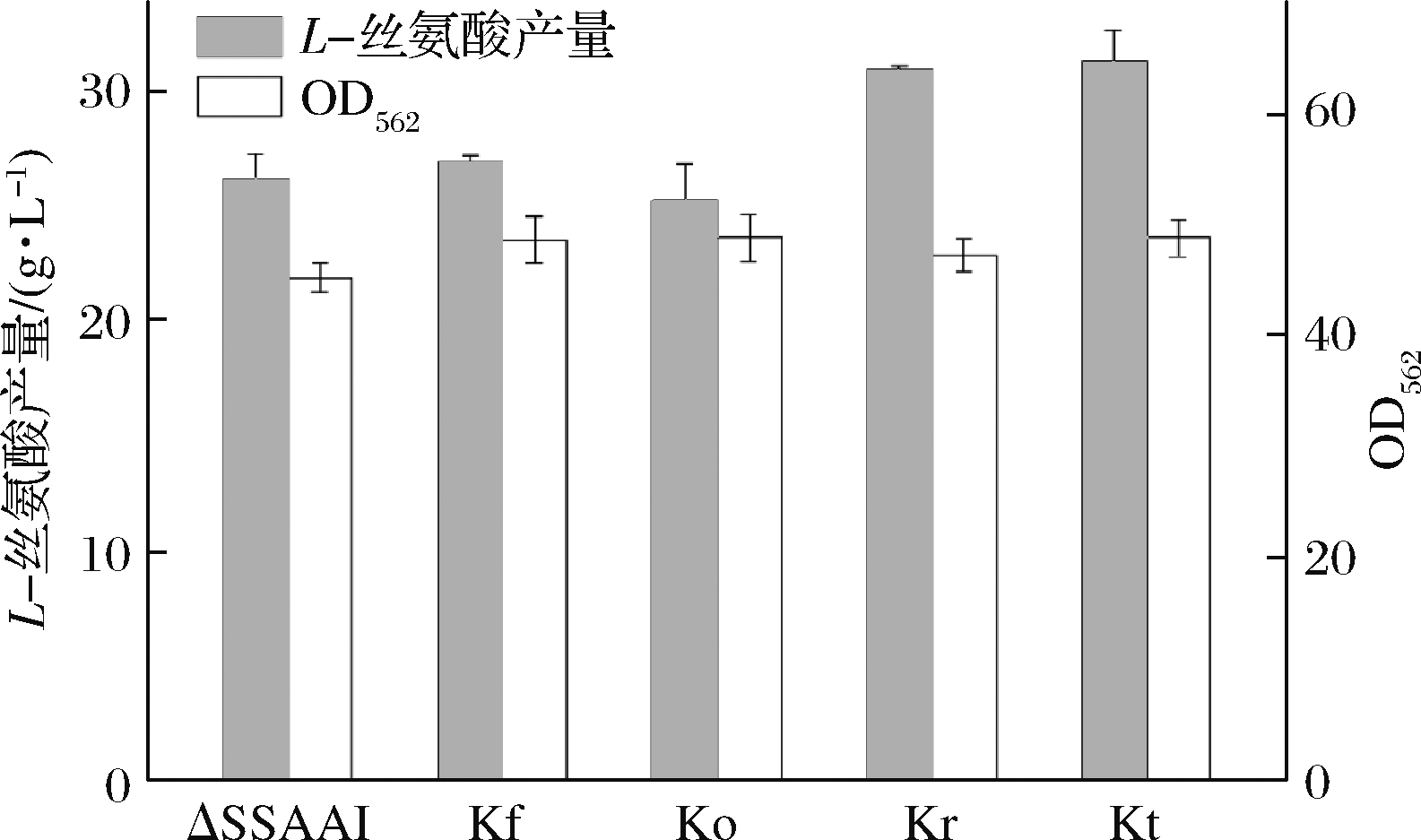
图2 基因突变对ΔSSAAI生长和产L-丝氨酸的影响
Fig.2 Effect of gene mutation on ΔSSAAI growth and
L-serine production
从图2可以看出,与出发菌株ΔSSAAI相比,4个突变株Kf、Ko、Kr和Kt的生物量均没有显著变化,突变菌株Kf、Ko的L-丝氨酸产量也没有显著变化;而突变菌株Kr、Kt的L-丝氨酸产量则有所提高,继而对L-丝氨酸产量提高的2株菌Kr、Kt做发酵过程分析,结果如图3和图4所示。

图3 出发菌株ΔSSAAI与回复突变菌株Kr、Kt生长
过程曲线
Fig.3 Growth curve of starting strain ΔSSAAI and
reverse-mutant strains Kr, Kt

图4 出发菌株ΔSSAAI与回复突变菌株Kr、Kt产
L-丝氨酸过程曲线
Fig.4 L-serine production curve of starting strain
ΔSSAAI and reverse-mutant strains Kr, Kt
从图3可以看出,Kr、Kt的生长趋势与出发菌株ΔSSAAI基本一致;从图4可以看出,发酵前期,Kr、Kt的L-丝氨酸产量与出发菌株ΔSSAAI相比变化不明显,而在发酵72 h后,2株突变株L-丝氨酸产量逐渐提高,最终突变菌株Kr、Kt的L-丝氨酸产量比出发菌株ΔSSAAI分别提高18.5%和18.3%。说明rhtA与tyrP基因突变可能与菌株产L-丝氨酸相关。接下来采用分别敲除和加强表达rhtA与tyrP研究这2个基因对菌株生长和产L-丝氨酸的影响。
2.2 rhtA、tyrP基因敲除对菌株生长和产L-丝氨酸的影响
采用1.2.1方法,利用pK18-R、pK18-T敲除质粒在ΔSSAAI上分别敲除rhtA和tyrP两个基因,敲除结果如图5、图6所示。图5泳道2与泳道1相比缺失了约800 bp,图6泳道2与泳道1相比缺失了约1 200 bp。表明基因rhtA和tyrP敲除菌株ΔrhtA与ΔtyrP构建成功。敲除菌株ΔrhtA与ΔtyrP发酵结果如图7所示,与出发菌株ΔSSAAI相比,基因敲除重组菌ΔrhtA与ΔtyrP中L-丝氨酸产量无显著变化。接下来通过加强表达rhtA与tyrP研究这2个基因对菌株生长和产L-丝氨酸的影响。
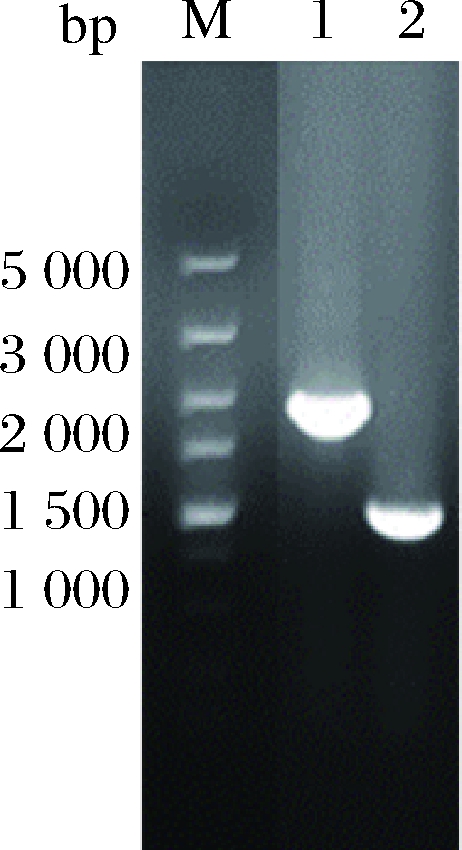
M-Marker;1-以ΔSSAAI基因组为模板扩增rhtA基因片段;
2-以敲除菌株基因组为模板扩增rhtA基因片段
图5 rhtA基因敲除电泳验证
Fig.5 rhtA gene knockout electrophoresis verification
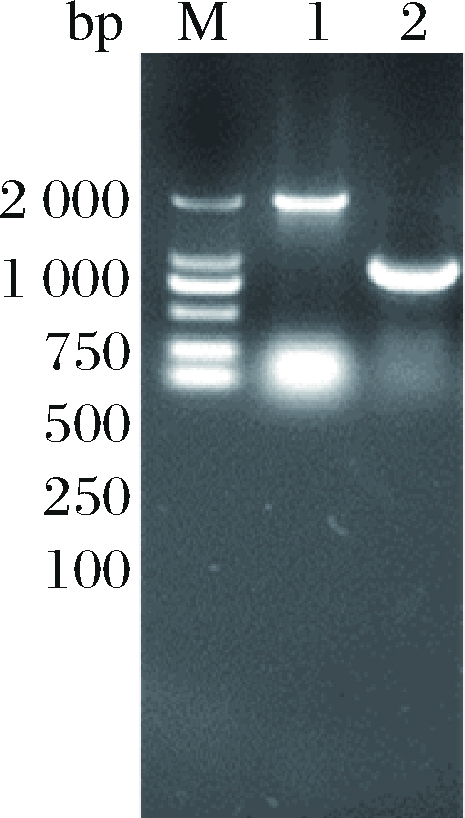
M-Marker;1-以ΔSSAAI基因组为模板扩增tyrP基因片段;
2-以敲除菌株基因组为模板扩增tyrP基因片段
图6 tyrP基因敲除电泳验证
Fig.6 tyrP knockout electrophoresis verification
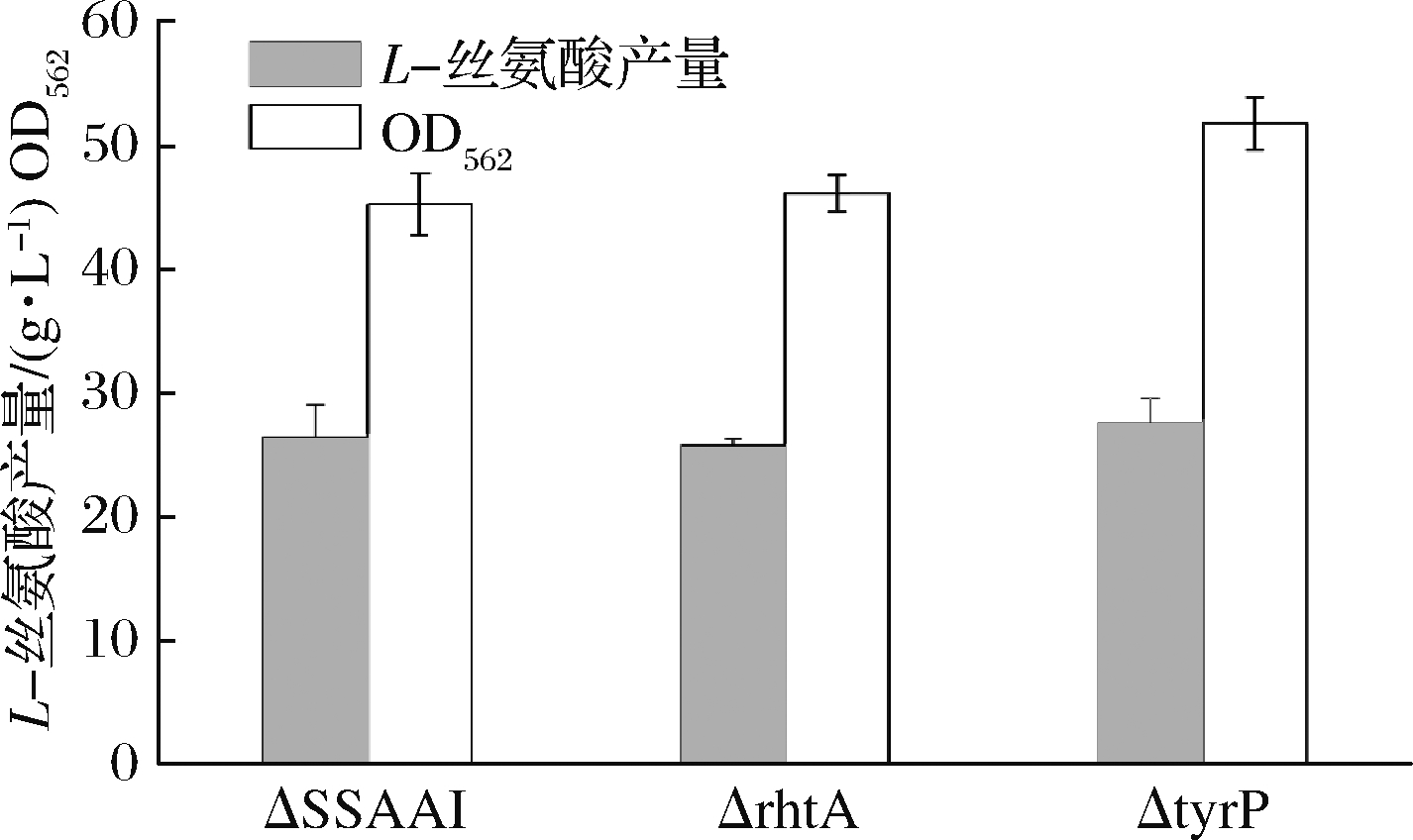
图7 rhtA、tyrP基因敲除对ΔSSAAI生长和
产L-丝氨酸的影响
Fig.7 Effect of rhtA, tyrP gene knockout on
ΔSSAAI growth and L-serine production
2.3 加强表达rhtA与tyrP基因对菌株生长和产L-丝氨酸的影响
在出发菌株ΔSSAAI中加强表达rhtA和tyrP,结果发现L-丝氨酸产量提高(结果未展示);接下来在高产L-丝氨酸菌株A36上加强表达这2个基因,研究基因的功能。采用诱导型质粒加强表达rhtA和tyrP得到重组菌株pM-R 和pM-T,考察加强表达rhtA与tyrP基因对菌株生长和产L-丝氨酸的影响,分别在发酵24、36、48、60 h采用1 mmol IPTG诱导pM-R和pM-T表达,结果如图8所示。
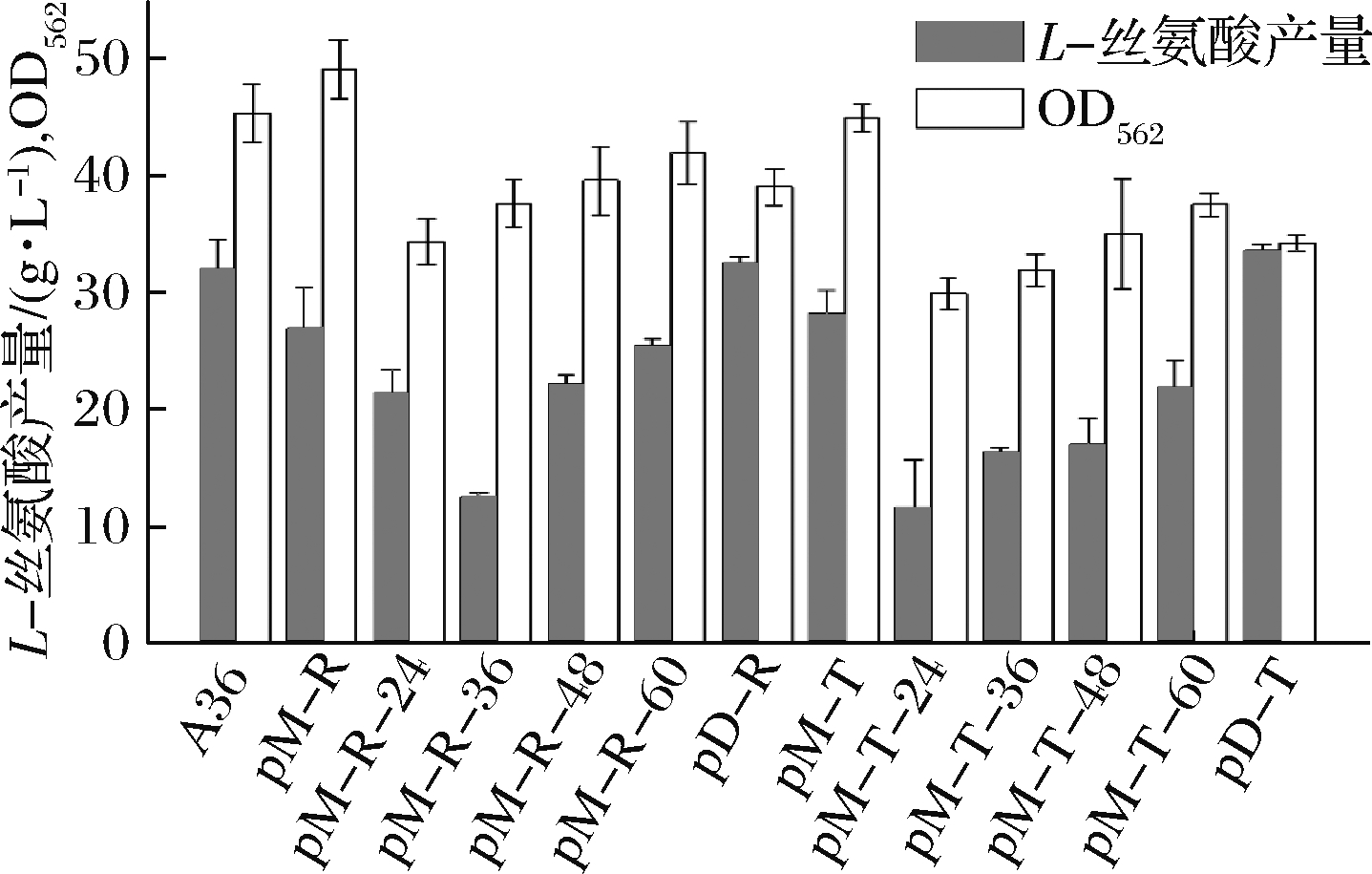
A36-对照菌株;pM-R、pM-R-24、pM-R-36、pM-R-48、
pM-R-60-不加IPTG和24、36、48、60 h加IPTG;pM-T、pM-T-24、
pM-T-36、pM-T-48、pM-T-60-不加IPTG和24、36、48、60 h加IPTG
图8 加强表达rhtA与tyrP对菌株生长和
产L-丝氨酸的影响
Fig.8 Effect of rhtA and tyrP overexpression on
the cell growth and L-serine production
从图8可以看出,加入IPTG时间越早,对重组菌株的生物量影响越大,最终菌株生物量低于对照菌株A36,推测是IPTG对菌株生长产生抑制的结果。然后采用组成型质粒构建了加强表达rhtA与tyrP的重组菌株pD-R和pD-T,发酵120 h,菌株的最大生物量分别为39.1和34.3,比对照菌株A36分别下降13.88%和24.5% (图9),L-丝氨酸产量分别达到32.55和33.7 g/L,比对照菌株A36分别提高6.5%和10.3%(图10),单位菌体产酸量分别提高17.74%和38.9%。说明加强表达rhtA与tyrP有利于菌株产L-丝氨酸,但不利于菌株生长。
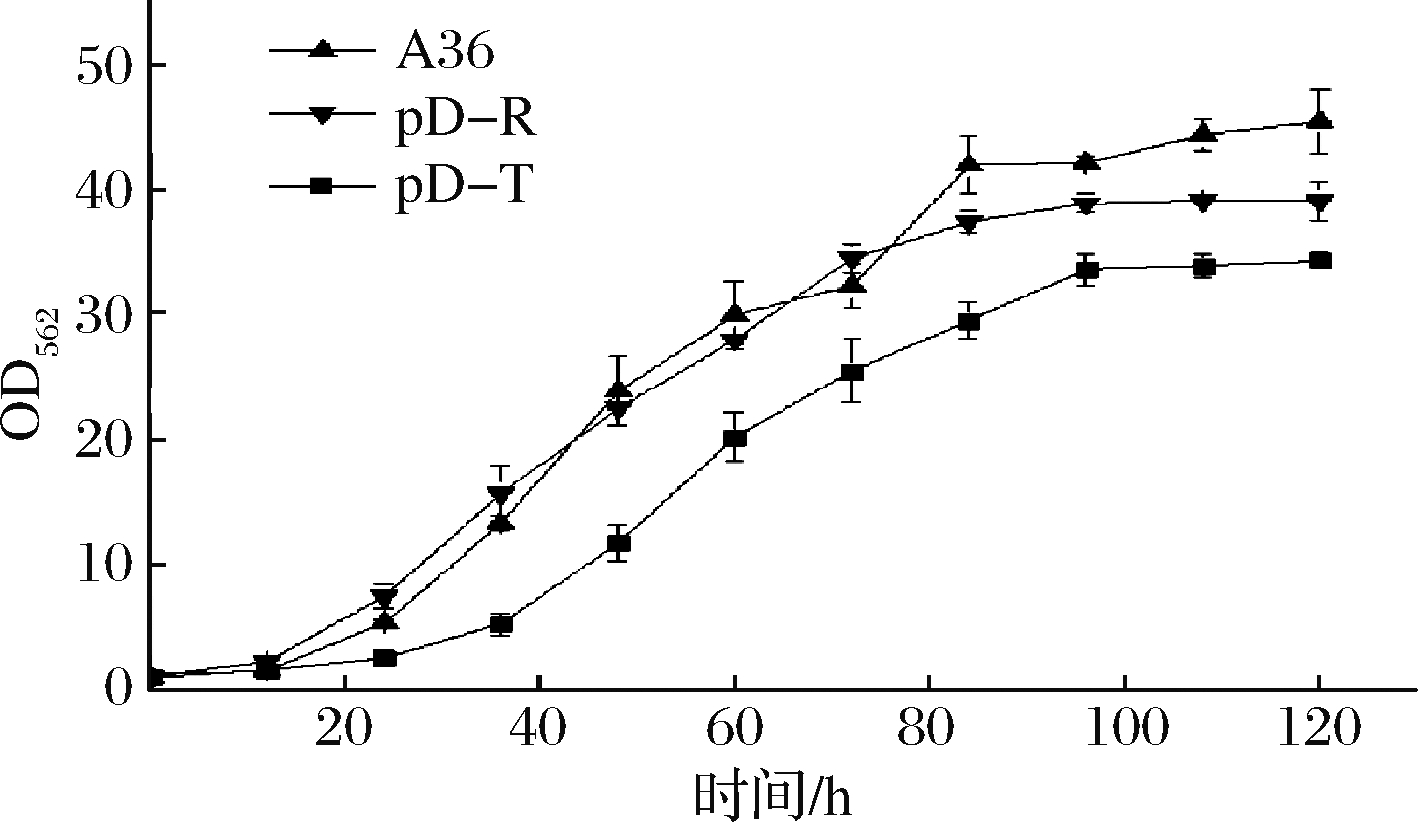
图9 出发菌株与加强表达rhtA与tyrP菌株
生长过程曲线
Fig.9 Growth curve of starting strains and strains
with overexpression of rhtA and tyrP
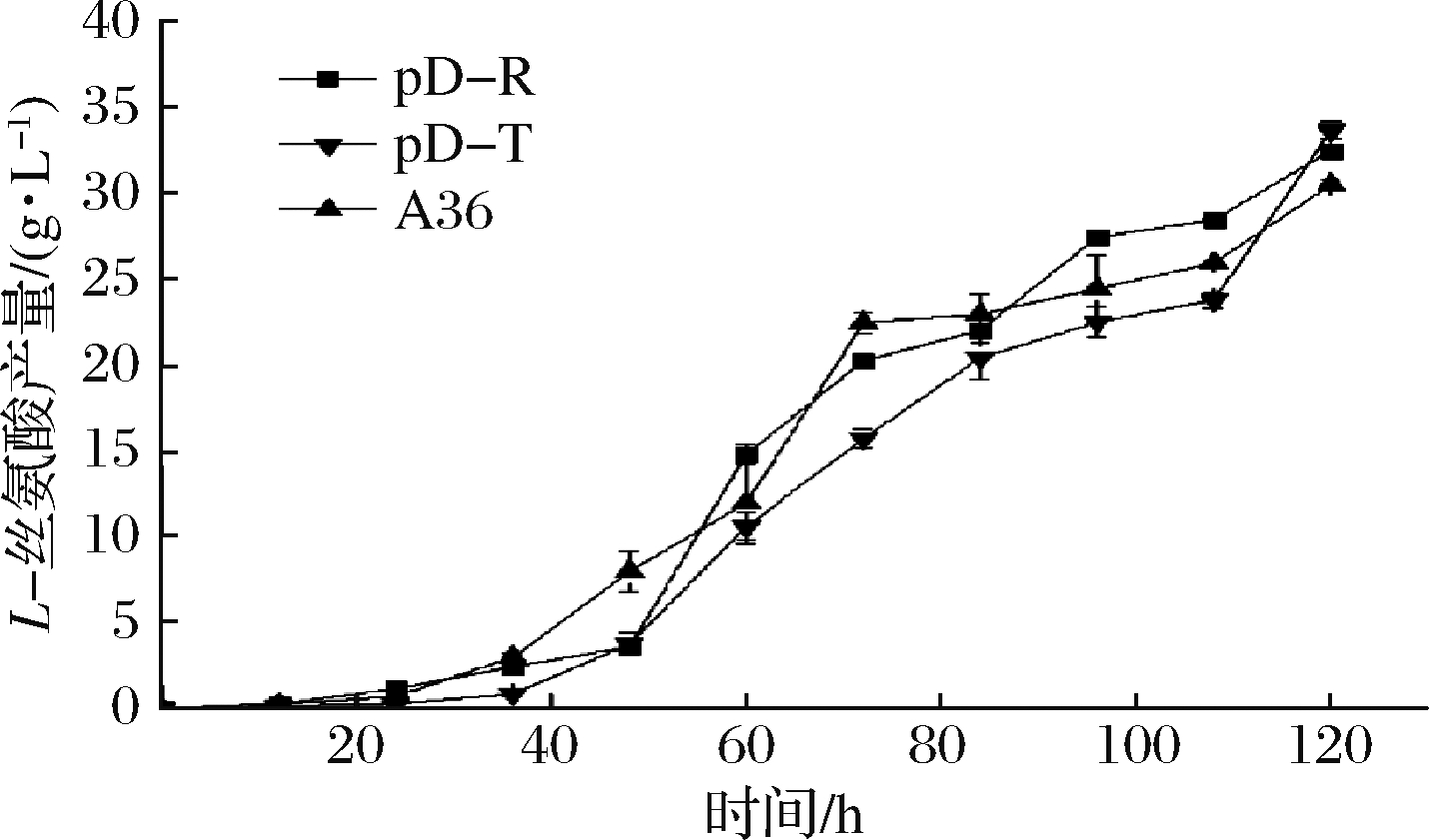
图10 出发菌株与加强表达rhtA与tyrP菌株
产L-丝氨酸过程曲线
Fig.10 L-serine production curve of starting strain and
strains with overexpression of rhtA and tyrP
3 讨论与结论
本课题组前期对产L-丝氨酸的谷氨酸棒杆菌ΔSSAAI进行ARTP诱变,得到1株生长与产L-丝氨酸均有提高的突变株A36。通过对ΔSSAAI 和A36进行全基因组测序以及比较基因组学分析,明确基因组遗传变异与菌株生长及产L-丝氨酸之间的关系,结果发现突变株A36中有11个基因发生氨基酸突变[15]。首先对其中与转运或代谢相关的4个差异基因进行研究,考察基因突变对ΔSSAAI产L-丝氨酸及生长的影响,结果表明,rhtA和tyrP的回复突变使得菌株ΔSSAAI产L-丝氨酸产量有提高,进一步采用基因敲除和加强表达研究rhtA和tyrP这2个基因对菌株生长和产L-丝氨酸的影响。结果表明,敲除rhtA或tyrP,对菌株的生长和产L-丝氨酸并无明显影响,而加强表达rhtA或tyrP则会导致产L-丝氨酸产量提高。继而在高产L-丝氨酸菌株A36上验证基因功能,结果发现,加强表达rhtA和tyrP的菌株L-丝氨酸积累量比A36分别提高6.47%和10.34%,但是加强表达rhtA和tyrP会影响菌株生长,尤其是在使用诱导型质粒加强表达时,菌株生物量大幅下降,并呈现加入IPTG越早,重组菌株的最终生物量越小的趋势,推测是菌株生长受到IPTG的抑制。研究结果表明,突变株A36中rhtA和tyrP基因突变有利于菌株产L-丝氨酸,敲除rhtA和tyrP对菌株产L-丝氨酸和生长影响不显著,而加强rhtA与tyrP有利于谷氨酸棒杆菌产L-丝氨酸不利于菌株生长。
C.glutamicum ATCC 13032全基因组测序及基因功能注释工作的完成[18],使得从基因组水平上解析谷氨酸棒杆菌高产L-丝氨酸机制成为可能,同时也提供了一种新的分子育种方法。而基于全基因组突变分析的分子育种策略,则能够从整个代谢网络角度分析突变与表型的关系,发现有利突变或突变组合;结合转录组学、蛋白质组学和代谢物组学等系统生物学方法的联合使用,找到改造靶点,实现对菌株的理性代谢改造[19-22]。OHNISHI等[23]利用比较基因组学的方法,比较分析了高产L-赖氨酸的谷氨酸棒杆菌和野生型菌株的基因组序列,鉴定到与L-赖氨酸积累相关基因的有益点突变;BECKER等[24]基于基因组学分析结果,以不产赖氨酸的野生型C. glutamicum ATCC 13032为出发菌株,依次对12个相关基因靶点进行敲除增加基因拷贝数及更换启动子等操作,使得重组菌株LYS12发酵30 h 赖氨酸产量即达到120 g/L。本课题组前期也曾对2株不同表型菌株做了全基因组测序分析,发现丙酮酸脱氢酶E1亚基编码基因aceE的突变与重组菌的副产物增加直接相关[16]。而本研究发现,rhtA和tyrP对谷氨酸棒杆菌生长和产L-丝氨酸均有影响,文献报道rhtA编码内膜转运蛋白RhtA,参与大肠杆菌中苏氨酸和高丝氨酸的外排[25],tyrP编码TyrP为酪氨酸特异性转运蛋白,功能是将酪氨酸转运到胞内[26]。从结果推测这2个基因编码的蛋白也可能与L-丝氨酸转运相关,加强表达rhtA和tyrP导致L-丝氨酸转运出胞增多,L-丝氨酸产量提高,同时流向菌株生长的碳代谢流减少,菌株生长受到影响。今后将对rhtA和tyrP编码的蛋白是否参与L-丝氨酸的转运进行研究,以及对另外7个突变点进行详细研究,明确基因型与表型之间的关系,为进一步理性设计改造L-丝氨酸高产菌株奠定基础。
[1] BECKER J, WITTMANN C. Systems and synthetic metabolic engineering for amino acid production the heartbeat of industrial strain development[J]. Current Opinion in Biotechnology, 2012, 23(5): 718-726.
[2] MUNDHADA H, SCHNEIDER K, CHRISTENSEN H B, et al. Engineering of high yield production of L-serine in Escherichia coli[J]. Biotechnology and Bioengineering, 2016, 113(4): 807-816.
[3] ZHANG Yun, SHANG Xiuling, LAI Shujun, et al. Reprogramming one-carbon metabolic pathways to decouple L-serine catabolism from cell growth in Corynebacterium glutamicum[J]. ACS Synthetic Biology, 2018, 7(2): 635-646.
[4] STOLZ M, PETERS-WENDISCH P, Etterich H, et al. Reduced folate supply as a key to enhanced L-serine production by Corynebacterium glutamicum[J]. Applied and Environmental Microbiology, 2007, 73(3): 750-755.
[5] CLOMBURG J M, CRUMBLEY A M, Gonzalez R. Industrial biomanufacturing: The future of chemical production[J]. Science, 2017, 355(6 320).
[6] 冯美卿, 曹秀格, 卢永辉. L-丝氨酸制备方法评述[J]. 氨基酸和生物资源, 2000, 22(3): 42-44.
[7] SHEN Peihong, CHAO Hongjun, JIANG Chenjian, et al. Enhancing production of L-serine by increasing the glyA gene expression in Methylobacterium sp.MB200[J]. Applied Biochemistry and Biotechnology, 2010, 160(3): 740-750.
[8] ZHANG Xiaomei, XU Guoqiang, SHI Jinsong, et al. Microbial production of, L-serine from renewable feedstocks[J]. Trends in Biotechnology, 2018, 36(7): 700-712.
[9] MUNDHADA H, SEOANE J M, SCHNEIDER K, et al. Increased production of L-serine in Escherichia coli through adaptive laboratory evolution[J]. Metabolic Engineering, 2017, 39: 141-150.
[10] PETERS-WENDISCH P, NETZER R, EGGELING L, et al. 3-Phosphoglycerate dehydrogenase from Corynebacterium glutamicum: The C-terminal domain is not essential for activity but is required for inhibition by L-serine[J]. Applied Microbiology and Biotechnology, 2002, 60(4): 437-441.
[11] PETERS-WENDISCH P, STOLZ M, ETTERICH H, et al. Metabolic engineering of Corynebacterium glutamicum for L-serine production[J]. Applied and Environmental Microbiology, 2005, 71(11): 7 139-7 144.
[12] 张晓娟, 窦文芳, 许泓瑜, 等. 维生素对谷氨酸棒杆菌SYPS-062直接发酵合成L-丝氨酸的影响[J]. 中国生物工程杂志, 2007, 27(5): 50-55.
[13] XU Guoqiang, ZHU Qinjian, LUO Yuchang, et al. Enhanced production of L-serine by deleting sdaA combined with modifying and overexpressing serA in a mutant of Corynebacterium glutamicum SYPS-062 from sucrose[J]. Biochemical Engineering Journal, 2015, 103: 60-67.
[14] ZHU Qinjian, ZHANG Xiaomei, LUO Yuchang, et al. L-serine overproduction with minimization of by-product synthesis by engineered Corynebacterium glutamicum[J]. Applied Microbiology and Biotechnology, 2015, 99(4): 1 665-1 673.
[15] ZHANG Xin, Zhang Xiaomei, XU Guoqiang, et al. Integration of ARTP mutagenesis with biosensor-mediated high-throughput screening to improve L-serine yield in Corynebacterium glutamicum[J]. Applied Microbiology and Biotechnology, 2018, 102(14): 5 939-5 951.
[16] GUO Wen, CHEN Ziwei, ZHANG Xiaomei, et al. A novel aceE mutation leading to a better growth profile and a higher L-serine production in a high-yield L-serine-producing Corynebacterium glutamicum strain[J]. Journal of Industrial Microbiology and Biotechnology, 2016, 43(9): 1 293-1 301.
[17] XU Daqing, TAN Yanzhen, SHI Feng, et al. An improved shuttle vector constructed for metabolic engineering research in Corynebacterium glutamicum[J]. Plasmid, 2010, 64(2): 85-91.
[18] KALINOWSKI J, BATHE B, BARTELS D, et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins[J]. Journal of Biotechnology, 2003, 104(1-3): 5-25.
[19] BENJAMIN F, ANSGAR P, CHRISTIAN T, et al. Quantitative proteomic overview on the Corynebacterium glutamicum L-lysine producing strain DM1730[J]. Journal of Proteomics, 2010, (73): 2 336-2 353.
[20] OUD B, MARIS A, DARAN J M, et al. Genome-wide analytical approaches for reverse metabolic engineering of industrially relevant phenotypes in yeast[J]. FEMS Yeast Research, 2012, 12(2): 183-196.
[21] HU Shiyuan, ZHENG Huajun, GU Yang, et al. Comparative genomic and transcriptomic analysis revealed genetic characteristics related to solvent formation and xylose utilization in Clostridium acetobutylicum EA 2018[J]. BMC Genomics, 2011, 12(1): 93.
[22] LEE C S, NAM J Y, SON E S, et al. Next-generation sequencing-based genome-wide mutation analysis of L-lysine-producing Corynebacterium glutamicum ATCC 21300 strain[J]. Journal of Microbiology, 2012, 50(5): 860-863.
[23] OHNISHI J, MITSUHASHI S, HAYASHI M, et al. A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant[J]. Applied Microbiology and Biotechnology, 2002, 58(2): 217-223.
[24] BECKER J, ZELDER O, H FNER S, et al. From zero to hero-Design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production[J]. Metabolic Engineering, 2011, 13(2): 159-168.
FNER S, et al. From zero to hero-Design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production[J]. Metabolic Engineering, 2011, 13(2): 159-168.
[25] LIVSHITS V A, ZAKATAEVA N P, ALESHIN V V, et al. Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli[J]. Research in Microbiology, 2003, 154(2): 123-135.
[26] 王钦, 曾伟主, 周景文. 大肠杆菌酪氨酸转运系统基因敲除对酪氨酸生产的影响[J]. 生物工程学报, 2019, 35(7): 1 247-1 255.