藜麦是一年生的藜科草本作物,原产于南美洲安第斯山脉[1]。它不仅富含蛋白质、矿物质和维生素,而且还含有较多的酚酸、黄酮和皂苷等植物化学物质[2]。因其营养均衡,美国国家航空航天局将藜麦誉为“最理想的太空食品”,联合国粮农组织将2013年作为“藜麦国际年”[3]。藜麦能够增强免疫力,同时还具有预防和辅助治疗肥胖、心血管疾病、糖尿病、和其他慢性疾病的功效[4-5]。除此之外,它具有极强的适应性(如耐旱、耐寒、耐盐碱等),可以在贫瘠的土壤上种植[6]。因藜麦营养成分丰富投资成本较低且可以代替低产量的粮食,我国于20世纪90年代展开藜麦种植,近几年将其选为产业扶贫项目之一[7-8]。目前已覆盖山西省、云南省、甘肃省、四川省与新疆等地区,且各省贫困县的产量已达到自给自足的水平[9]。藜麦在食品加工过程中会产生大量的麸皮,这些麸皮通常会被焚烧或者丢弃,从而影响生态环境。然而,麸皮中含有大量的皂苷,可利用麸皮提取皂苷以达到节约资源和提高经济效益的目的。
皂苷又称皂素、皂体等,是一种以三萜或螺旋甾烷类化合物为苷元的,结合不同糖苷形成的化合物[10]。组成皂苷结构的糖多为葡萄糖、葡萄糖醛酸、半乳糖、半乳糖醛酸、木糖、鼠李糖和阿拉伯糖等[11-12]。根据苷元结构不同可分为甾体皂苷和三萜皂苷,其苷元可含有—OH、—COOH、—CH3等官能团。因此,官能团的数量、糖链种类和数量的不同造成皂苷结构多样性。藜麦皂苷是藜麦糖基化的次生代谢物,属于三萜糖苷类化合物,分布于整个植株内(如叶子、花、果实、种子和种皮),但主要存在于种皮的乳突细胞中,可以抵御鸟类和虫子的捕食[13]。皂苷含量会随着植株的品种和种植条件的变化而变化,即使在同株植物的不同部位皂苷含量和种类亦有差异,总含量占粒重2%~6%[14]。
近年来,随着分离技术和鉴定技术的迅速发展,对藜麦皂苷的研究越来越多,主要是从提取工艺、化学结构以及生物活性3个方面展开研究。皂苷易吸潮,易溶于热水、甲醇、正丁醇以及乙醇,不溶于氯仿、丙酮、乙醚和苯。一般采用甲醇或乙醇提取,通过正丁醇萃取以获得粗皂苷。由于皂苷降低了藜麦的适口性和消化率,通常用研磨或洗涤减少皂苷含量,以提供更好的口感[15-16]。因此植物学家培育出皂苷含量小于鲜重0.11%的甜藜,以减少上述工艺带来的损失[17]。
皂苷是天然的表面活性剂,不仅用于医药行业,还广泛应用于化妆品、饮料等行业。皂苷在信号转导和细胞过程也有很大的影响,最显著的作用是改变细胞膜通透性,它可以作为表面活性分子与脂质和胆固醇膜相互作用,形成不稳定的孔隙来破坏膜结构[18]。并影响酶活性、氧化还原、细胞器完整性等,例如通过激活细胞程序性死亡来干扰细胞[19-20]。
1 藜麦皂苷化学研究
目前,从藜麦中分离的皂苷是由C-3和C-28结合在疏水苷元上的亲水性低聚糖组成的三萜糖苷,主要的糖是葡萄糖(glucose,Glc)、半乳糖(galactose,Gal)和阿拉伯糖(arabinose,Ara),而葡萄糖醛酸(glucuronic anid,GlcA)和木糖(xylose,Xyl)很少见[21]。根据苷元连接的糖链多少,分为单糖链、双糖链和三糖链皂苷。近年来,发现藜麦皂苷的糖苷配基主要有齐墩果酸,常春藤皂苷元,美商陆酸,serjanic酸,spergulagenic酸,gypsogenin(3β-hydroxy-23-oxo-olean-12-en-28-oic acid), 3β-hydroxy-27-oxo-olean-12-en -28-oic acid和3β,23α, 30β-trihydroxy-olean-12-en-28-oic acid,所有这些都衍生于三萜皂苷β-香草素[22],其化学结构见图1。
1992年CHAUHANG等[23]研究并测定了藜麦壳中皂苷的含量,结果表明在藜麦壳中存在34%的皂苷,因此脱壳会除去大量的皂苷,进而提高藜麦食用时口感。
CUADRADO等[24]使用快原子轰击-质谱与气相色谱联用技术对白藜和灰藜籽实的皂苷提取物进行了分析鉴定,研究发现齐墩果酸、常春藤皂苷、美商陆酸以及脱氧美商陆酸是藜麦皂苷的主要类型,齐墩果酸占比较大,且灰藜比白藜皂苷中的齐墩果酸含量要高,这与CHAUHANG[23]的研究结果一致。
2000年,MASTEBROEK等[25]检测了甜藜和苦藜叶子和籽实中的皂苷含量,甜藜籽实中的皂苷含量为0.2~0.4 g/kg干重。2019年LIM等[26]对藜麦各个部分的总皂苷含量进行了测量,结果表明藜麦根中总皂苷含量最高(13.3 g/100g),其次是藜麦麸皮、藜麦茎、藜麦种子皮和藜麦叶。
目前为止,在藜麦中检测得到87种皂苷,其中逾40种已被鉴定和报道。由表1 可知近年来检测出的藜麦皂苷类型以及取代基等。2001年,WOLDEMICHAEL等[27]主要分离得到6种皂苷,通过核磁共振进行鉴定,其中已报道皂苷见表1(序号7、12、13、22、24),新发现皂苷见表1(序号19),并采用质谱法再鉴定出至少16种皂苷。同年,DINI等[28]使用核磁共振和电喷雾质谱法(electrospray ionization mass spectrometry, ESI-MS)对从藜麦种子中分离出的皂苷进行结构鉴定,共鉴定6种皂苷,其中新发现的2个美商陆皂苷见表1(序号21、23),新发现的2个齐墩果烷皂苷见表1(序号5、6),还有2种为已报道的美商陆皂苷。除此之外,DINI[29]从藜麦籽实中分离出6种三萜皂苷见表1(序号12、18、20、30、37、38),其中序号18和38是首次报道的皂苷。
2002年ZHU等[30]从藜麦籽实中分离得到12种皂苷,经核磁共振鉴定其结构,其中有3种皂苷首次从藜麦中分离,见表1(序号1、10、41)。
2006年MADL等[21]使用纳米电喷雾多级串联质谱对藜麦籽实中的皂苷粗提物进行检测,共检测出87种皂苷,其中有68种为新检测到的化合物。
2008年KUJANABHAGAVAD等[31]从藜麦不同部位(花、种皮、果实、籽实等)中,共提取分离出20种皂苷,其中有16种皂苷从藜麦花和果实中首次分离并报道,包括2个serjanic酸双糖链皂苷,4个齐墩果酸双糖链皂苷,4个双糖链常春藤皂苷元,5个商陆酸双糖链皂苷和1个双糖链皂苷。
2012年VERZA等[32]利用大孔吸附树脂进行富集获得2个藜麦粗皂苷组分(FQ70和FQ90),并用超高效液相色谱-四级杆飞行时间质谱联用(ultra-performance liquid chromatography-quadrupole-time-of-flight-mass spectrometry,UPLC/Q-TOF-MS)从中检测到10种皂苷。2个组分中有8种三萜皂苷同时被检测到,而剩下2种皂苷仅在FQ90中检测到,分别属于齐墩果酸型皂苷和常春藤型皂苷。
2017年ESCRIBANO等[33]用高效液相色谱飞行质谱联用仪对红紫色品种中的皂苷进行了鉴定,发现5种藜麦皂苷,见表1(序号7、8、16、24、31)。
实验室前期发现,国产灰藜中主要有6种皂苷,见表1(序号12、17、22、24、27、29)。主要以β-D-glc(1-3)-α-L-ara-β-D-glc-Phytolaccagenic含量最高。藜麦在收获后会被自身酶水解掉糖链,如表1中的序号29(α-L-ara)就是在序号24(α-L-ara-β-D-glc)的基础上被水解掉了1个葡萄糖,这可能是造成各个学者检测到不同种类的皂苷的原因之一[16]。

A-齐墩果酸;B-常青藤皂苷元;C-美商陆酸;D-serjanic酸;E-spergulagenic酸;F-gypsogenin;G-3β-hydroxy-27-oxo-12-enacid; H-3β,23α,30β-trihydroxy-olean-12-en-28-oic-acid
图1 藜麦皂苷的化学结构
Fig.1 Chemical structure of quinoa saponins
表1 藜麦中的三萜皂苷
Table 1 Triterpene saponins in quinoa
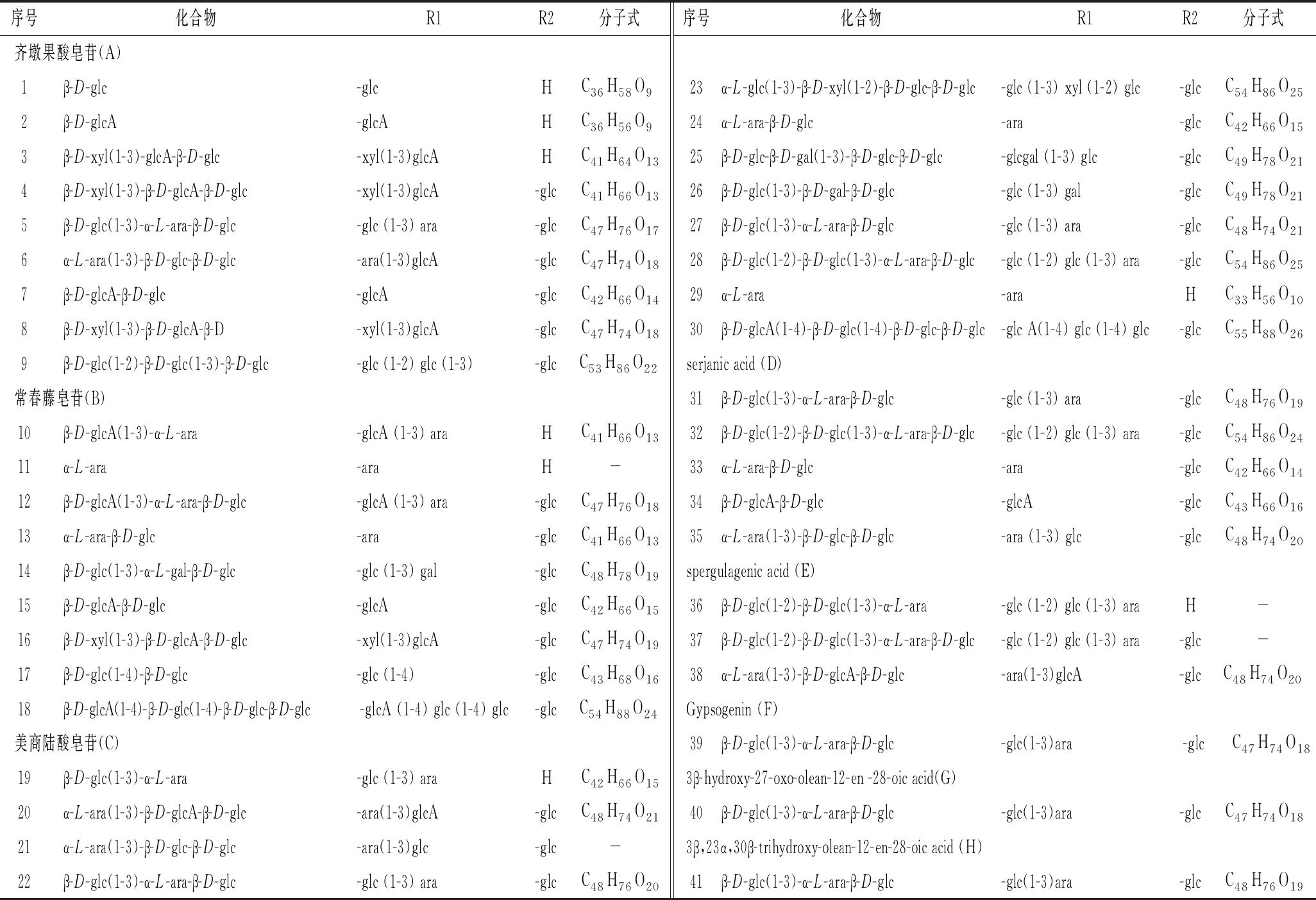
序号化合物R1R2分子式序号化合物R1R2分子式齐墩果酸皂苷(A)1β-D-glc-glcHC36H58O923α-L-glc(1-3)-β-D-xyl(1-2)-β-D-glc-β-D-glc-glc (1-3) xyl (1-2) glc-glcC54H86O252β-D-glcA-glcAHC36H56O924α-L-ara-β-D-glc-ara-glcC42H66O153β-D-xyl(1-3)-glcA-β-D-glc-xyl(1-3)glcAHC41H64O1325β-D-glc-β-D-gal(1-3)-β-D-glc-β-D-glc-glcgal (1-3) glc-glcC49H78O214β-D-xyl(1-3)-β-D-glcA-β-D-glc-xyl(1-3)glcA-glcC41H66O1326β-D-glc(1-3)-β-D-gal-β-D-glc-glc (1-3) gal-glcC49H78O215β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-3) ara-glcC47H76O1727β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-3) ara-glcC48H74O216α-L-ara(1-3)-β-D-glc-β-D-glc-ara(1-3)glcA-glcC47H74O1828β-D-glc(1-2)-β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-2) glc (1-3) ara-glcC54H86O257β-D-glcA-β-D-glc-glcA-glcC42H66O1429α-L-ara-araHC33H56O108β-D-xyl(1-3)-β-D-glcA-β-D-xyl(1-3)glcA-glcC47H74O1830β-D-glcA(1-4)-β-D-glc(1-4)-β-D-glc-β-D-glc-glc A(1-4) glc (1-4) glc-glcC55H88O269β-D-glc(1-2)-β-D-glc(1-3)-β-D-glc-glc (1-2) glc (1-3)-glcC53H86O22serjanic acid (D)常春藤皂苷(B)31β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-3) ara-glcC48H76O1910β-D-glcA(1-3)-α-L-ara-glcA (1-3) araHC41H66O1332β-D-glc(1-2)-β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-2) glc (1-3) ara-glcC54H86O2411α-L-ara-araH -33α-L-ara-β-D-glc-ara-glcC42H66O1412β-D-glcA(1-3)-α-L-ara-β-D-glc-glcA (1-3) ara-glcC47H76O1834β-D-glcA-β-D-glc-glcA-glcC43H66O1613α-L-ara-β-D-glc-ara-glcC41H66O1335α-L-ara(1-3)-β-D-glc-β-D-glc-ara (1-3) glc-glcC48H74O2014β-D-glc(1-3)-α-L-gal-β-D-glc-glc (1-3) gal-glcC48H78O19spergulagenic acid (E)15β-D-glcA-β-D-glc-glcA-glcC42H66O1536β-D-glc(1-2)-β-D-glc(1-3)-α-L-ara-glc (1-2) glc (1-3) araH-16β-D-xyl(1-3)-β-D-glcA-β-D-glc-xyl(1-3)glcA-glcC47H74O1937β-D-glc(1-2)-β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-2) glc (1-3) ara-glc-17β-D-glc(1-4)-β-D-glc-glc (1-4)-glcC43H68O1638α-L-ara(1-3)-β-D-glcA-β-D-glc-ara(1-3)glcA-glcC48H74O2018β-D-glcA(1-4)-β-D-glc(1-4)-β-D-glc-β-D-glc-glcA (1-4) glc (1-4) glc-glcC54H88O24Gypsogenin (F)美商陆酸皂苷(C)39β-D-glc(1-3)-α-L-ara-β-D-glc-glc(1-3)ara-glcC47H74O1819β-D-glc(1-3)-α-L-ara-glc (1-3) araHC42H66O153β-hydroxy-27-oxo-olean-12-en -28-oic acid(G)20α-L-ara(1-3)-β-D-glcA-β-D-glc-ara(1-3)glcA-glcC48H74O2140β-D-glc(1-3)-α-L-ara-β-D-glc-glc(1-3)ara-glcC47H74O1821α-L-ara(1-3)-β-D-glc-β-D-glc-ara(1-3)glc-glc -3β,23α,30β-trihydroxy-olean-12-en-28-oic acid (H)22β-D-glc(1-3)-α-L-ara-β-D-glc-glc (1-3) ara-glcC48H76O2041β-D-glc(1-3)-α-L-ara-β-D-glc-glc(1-3)ara-glcC48H76O19
注:“-”表示无,“R1,R2”表示取代基
2 藜麦皂苷的生物活性
2.1 抗氧化
LETELIER等[35]在藜麦籽粒水醇提取物中发现藜麦除了含有多酚之外还有硫醇化合物,并对其成分和抗氧化性进行了研究。结果表明,水醇提取物中存在三萜烯皂苷并可以抑制由Cu2+/抗坏血酸促进的微粒体脂质过氧化过程,10 mL和20 mL的水醇提取物剂量可以防止Cu2+/抗坏血酸促进的微粒体硫醇含量的损失,分别为50%和100%。NSIMBA等[36]通过铁还原/抗氧化能力和1,1-二苯基-2-三硝基苯肼(1,1-diphenyl-2-picrylhydrazyl,DPPH)自由基清除能力对不同品种藜麦种子的正丁醇提取物的抗氧化活性进行了研究,结果表明该提取物具有高效的抗氧化活性,可用于延缓或限制脂质氧化。杜婧婷等[37]对藜麦种皮中的皂苷进行了还原力测定、清除超氧阴离子自由基能力、清除羟自由基能力以及清除DPPH 自由基能力测定。4个实验均表明藜麦种皮皂苷具有抗氧化能力,且清除率与皂苷质量浓度呈正相关。2019年LIM[26]测定了藜麦不同位置的皂苷含量并分析了其抗氧化活性,结果表明藜麦根中皂苷含量最高且具有较高的抗氧化活性。
2.2 抗菌活性
藜麦粗皂苷在50 μg/mL质量浓度下对白色念珠菌有抑制作用,单体皂苷却没有表现出明显的抗菌作用,说明不同的皂苷之间具有协同作用。在分离的皂苷中,单糖链和双糖链美商陆酸型皂苷具有抗菌活性,最低抑菌浓度值在100~500 μg/mL[27]。
STUARDO等[38]使用6种不同的提取方法获得粗皂苷并评价其是否对灰霉病菌具有抗菌活性。提取方法分别为:未纯化、纯化、未纯化碱处理、纯化碱处理、未热处理纯化碱处理和未热处理未纯化碱处理。结果表明,未经处理的藜麦提取物对灰霉病菌菌丝生长的抑制活性最低。即使在7 mg/mL的质量浓度下,也没有抑制分生孢子萌发。而皂苷提取物经碱处理后,菌丝生长和分生孢子萌发受到明显抑制。当质量浓度为5 mg/mL时,分生孢子萌发抑制率为100%,即使在96 h后也是如此。基于荧光染料SYTOX绿摄取的真菌膜完整性实验表明,碱处理的皂苷会引起膜的破坏,而未处理的皂苷则没有影响。碱处理的皂苷具有较高的抗真菌活性,这可能是由于形成了与细胞膜中的甾醇具有更高亲和力的疏水性皂苷衍生物。
SUN等[39]通过AB-2树脂分离藜麦皂苷(quinoa saponin,QS)和碱转化皂苷(alkalitransformed saponin,ATS)得到QS-30,QS-80,ATS-30和ATS-80,并评价这4种皂苷是否对口臭相关细菌(牙龈卟啉单胞菌、产气荚膜梭菌和核梭杆菌)有抑菌活性。结果表明QS-80和ATS-80具有抑菌活性。而且经碱处理的皂苷比原始的藜麦皂苷具有更高的抑菌活性,原因是ATS-80中具有极性较低的皂苷,使其更容易与细菌细胞膜相互作用,破坏细胞膜的完整性,降低膜电位。
DONG等[34]测定了分离出的皂苷对金黄色葡萄球菌、表皮葡萄球菌、蜡状芽孢杆菌、肠炎沙门氏菌、铜绿假单胞菌和伊万诺韦李斯特菌的抑菌效果。结果表明6种单体皂苷对金黄色葡萄球菌、表皮葡萄球菌和蜡状芽孢杆菌均有抑菌作用。α-L-ara-β-D-glc-phytolaccagenic对金黄色葡萄球菌和表皮葡萄球菌的抑菌活性最强,最低抑菌浓度为0.062 5 mg/mL,最低杀菌浓度为0.125 mg/mL,且抑菌效果随着皂苷浓度的增加而增加。
2.3 抗炎活性
从藜麦分离得到的皂苷不仅可以降低炎症介质的产生还能抑制炎症细胞因子的释放[40]。在大鼠身上进行的一项研究表明,定期食用藜麦可以降低大鼠脂肪组织和肠道内层的炎症程度。植物皂苷的抗炎特性可作为药物的活性成分。藜麦皂苷可抑制脂多糖诱导小鼠巨噬细胞(RAM264.7)产生炎症介质NO,并抑制肿瘤坏死因子-α和白细胞介素-6等炎性细胞因子的释放[41]。这些结果均表明藜麦皂苷可用于预防和治疗炎症。
2.4 免疫活性
近年来,越来越多的研究集中在以植物皂苷为基础的新型免疫佐剂,目的是为动物和人类寻找更安全、更有效的疫苗,这可能会提高现有疫苗的有效性[42-44]。VERZA等[32]研究分离得到的2种藜麦粗皂苷FQ70和FQ90对卵清蛋白(ovalbumin,OVA)免疫小鼠体液免疫和细胞免疫反应的辅助作用。经检测,二者均可以有效的增加OVA免疫小鼠血清中的抗体(免疫球蛋白G和免疫球蛋白A),但FQ70的溶血活性相对较低,因此制作免疫佐剂应该选择具有明显辅助作用且溶血活性相对较低的FQ70。在FQ70中的8种皂苷多数为单糖苷,这与WOLDEMICHAEL等[27]之前报道的一致,单糖苷的溶血活性明显高于双糖苷。
2.5 杀螺活性
藜麦皂苷具有杀螺活性,且这种活性在经过碱性处理后会显著增强。研究证实经过碱处理的皂苷对福寿螺具有100%的致死率,且用量比商业灭螺剂(氯硝柳胺)低。结果表明,经碱处理后双糖链皂苷会转化成活性更高的单糖链皂苷。在测试最高浓度时,该产品没有对金鱼或者罗非鱼表现出毒性[45-46]。RUIZ[47]制作了一种以藜壳皂苷为改良剂的新型杀螺药剂,并在鄂尔多河三角洲地区进行了斑点波马科防治效果的实验。结果表明,以藜壳皂苷为基础的新型杀螺药剂对斑点波马科幼虫的杀灭效果较好,在实验室和野外条件下均有较好的防治效果。在实验室条件下,皂甙含量在7 mg/mL以上时,72 h杀灭率达100%,在3个稻田进行的田间试验表明,剂量分别为6.5、7.2和7.7 mg/mL时,96 h后杀灭率分别为88%、89%和93%。同时做了毒理学实验,结果表明,该产品对水环境高度安全且可能成为一种安全防治稻田斑点拟线虫的产品。
2.6 其他功效
藜麦皂苷除上述功能外,还有抑制脂肪、溶血、抗癌、抗疲劳、调节脂质代谢、预防血管疾病、降血脂、保护肝脏、清除亚硝酸盐、抗病毒、促进药物吸收等生物活性。除此之外,在食品工业中藜麦皂苷还被用作添加剂、风味调节剂和防腐剂[48-50]。实验室前期发现,藜麦皂苷口服可能对小鼠胃肠道产生刺激性,引起小鼠腹泻,这可能与皂苷在体内水解后产生刺激性强的低级性皂苷有关[16]。
3 结论
皂苷存在于100多个植物家族中,其中至少有150种天然皂苷具有显著的抗癌活性。近年来,由于皂苷的结构多样性及其具有的多种生物活性,使其成为研究热点。藜麦皂苷具有结构类型丰富,植物体含量高,毒性较低等优点。更重要的是,藜麦皂苷多存在于茎叶、麸皮中,属于加工废料,价格低廉。如果进行回收利用则可以大大提高藜麦的经济效益。目前,行业内已有企业以藜麦皂苷为活性物质用于生物农药,化妆品的生产。鉴于藜麦皂苷独特的资源与价格优势,对其开发利用不但可以大大降低研发成本,还可以提高藜麦的经济效益。
[1] LI G T, ZHU F.Quinoa starch:Structure, properties, and applications[J].Carbohydrate Polymers, 2018, 181: 851-861.
[2] GUERRERO J B S, RODRIGUEZ D J D, GARCIA R R.Quinoa saponins:Concentration and composition analysis[C].Trends in New Crops & New Uses Fifth National Symposium,2002.
[3] NASCIMENTO A C, MOTA C, COLHO I, et al.Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina:Proximates, minerals and trace elements[J].Food Chemistry, 2014, 148:420-426.
[4] TANG Y, TSAO R.Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects:A review[J].Molecular Nutrition & Food Research, 2017, 61(7):1-16.
[5] VARLI S N, SANLIER N.Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.)[J].Journal of Cereal Science, 2016, 69:371-376.
[6] 邓俊琳, 夏陈, 张盈娇, 等.拉萨藜麦的营养成分分析与比较[J].中国食物与营养, 2017, 23(9):55-58.
DENG J L, XIA C, ZHANG Y J, et al.Nutrition composition analysis on quinoa cultivated in Lasa[J].Food and Nutrition in China, 2017, 23(9):55-58.
[7] 贾金才, 杨颖辉, 王亚荣.张北县藜麦产业发展现状分析[J].农业与技术, 2020, 40(5):29-30.
JIA J C, YANG Y H, WANG Y R.Analysis on the development status of quinoa industry in Zhangbei county[J].Agriculture and Technology, 2020, 40(5):29-30.
[8] 徐骋. 基于藜麦的种植技术与营养价值分析[J].农家参谋, 2019(20):71.
XU P.Planting technology and nutritional value analysis based on quinoa[J].The Farmers Consultant, 2019(20):71.
[9] 梁军林, 李霞, 李嘉奕, 等.藜麦产品研发现状及前景[J].粮食加工, 2017, 42(6):64-67.
LIANG J L, LI X, LI J Y, et al.Development status and prospects of quinoa products[J].Grain Processing, 2017, 42(6):64-67.
[10] USTUNDAG O Z, MAZZA G.Saponins:Properties, applications and processing[J].Critical Reviews in Food Science and Nutrition, 2007, 47(3):231-258.
[11] PICKETT J A, KHAN Z R.Plant volatile-mediated signalling and its application in agriculture:Successes and challenges[J].New Phytologist, 2016, 212(4):856-870.
[12] MITHOFER A, BOLAND W, MAFFEI M E. Annual Plant Reviews, Molecular Aspects of Plant Disease Resistance[M]. Chichester: Wiley-Blackwell, 2009:261-291.
[13] QASIM M, ISLAM W, JAVARIA H, et al. Co-Evolution of Secondary Metabolites[M]. Switzerland: Springer, 2019:898-911.
[14] JARVIE D E, HO Y S, LIGHTFOOT A J, et al.The genome of Chenopodium quinoa[J].Nature,2017, 542(7 641):307-312.
[15] JURADO J F, POLLIER J, MOSES T, et al.Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves[J].Plant Science, 2016, 250:188-197.
[16] XUE P, ZHAO L, WANG Y J, et al.Reducing the damage of quinoa saponins on human gastric mucosal cells by a heating process[J].Food Science & Nutrition, 2020, 8(1):500-510.
[17] JURADO J F, POLLIER J, MOSES T, et al.Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves[J].Plant Science, 2016,250:188-197.
[18] CHWALEK M, LALUN N, HELENE B, et al.Structure-activity relationships of some hederagenin diglycosides:Haemolysis, cytotoxicity and apoptosis induction[J].Biochimica Et Biophysica Acta General Subjects, 2006, 1760(9):1 418-1 427.
[19] HO S S, PAL S.Margarine phytosterols decrease the secretion of atherogenic lipoproteins from HepG2 liver and Caco2 intestinal cells[J].Atherosclerosis, 2005, 182(1):29-36.
[20] SOLTANI M, PARIVAR K, BAHARARA J, et al.Hemolytic and cytotoxic properties of saponin purified from Holothuria leucospilota sea cucumber[J].Reports of Biochemistry & Molecular Biology, 2014, 3(1):43-50.
[21] MADL T, STERK H, MITTELBACH M, et al.Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa[J].Journal of the American Society for Mass Spectrometry, 2006, 17(6):795-806.
[22] MUIR A D, BALLANTYUE K D, HALL T W. Saponins in Food, Feedstuffs and Medicinal Plants[M]. Oxford and London: Clarendon Press, 2000:35-41.
[23] CHAUHANG S, ESKINN A M, TKACHUK R.Nutrients and antinutrients in quinoa seed[J].Cereal Chemistry, 1992, 69(1):85-88.
[24] CUADRADO C, AYET G, BURBANO C, et al.Occurrence of saponins and sapogenols in Andean crops[J].Journal of the Science of Food & Agriculture, 1995, 67(2):169-172.
[25] MASTEBROEK H D, LIMBURG H, GILLES T, et al.Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd)[J].Journal of the Science of Food and Agriculture, 2000, 80(1):152-156.
[26] LIM J G, PARK H M, YOON K S.Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.)[J].Food Science & Nutrition, 2020, 8(1):694-702.
[27] WOLDEMICHAEL G M, WINK M.Identification and biological activities of triterpenoid saponins from Chenopodium quinoa[J].Journal of Agricultural & Food Chemistry, 2001:49(5):2 327-2 332.
[28] DINI I, SCHETTINO O, SIMIOLI T, et al.Studies on the constituents of Chenopodium quinoa Seeds: Isolation and characterization of new triterpene saponins[J].Journal of Agricultural & Food Chemistry, 2001, 49(2):741-746.
[29] DINI I, TENORE G C, SCHETTINO O, et al.New oleanane saponins in Chenopodium quinoa[J].Journal of Agricultural and Food Chemistry, 2001, 49(8):3 976-3 981.
[30] ZHU N Q, SHENG S Q, SANG S M, et al.Triterpene saponins from debittered quinoa (Chenopodium quinoa) seeds[J].Journal of Agricultural & Food Chemistry, 2002, 50(4):865-867.
[31] KUJANABHAGAVAD T, THONGPHASUK P, CHAMULITRAT W, et al.Triterpene saponins from Chenopodium quinoa Willd[J].Phytochemistry, 2008, 69(9):1 919-1 926.
[32] VERZA S G, SILVERIRA F, CIBULSKI S, et al.Immunoadjuvant activity, toxicity assays, and determination by UPLC/Q-TOF-MS of triterpenic saponins from Chenopodium quinoa seeds[J].Journal of Agricultural & Food Chemistry, 2012, 60(12):3 113-3 118.
[33] ESCRIBANO J, CABANES J, ATIRNZAR M J, et al.Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties[J].Food Chemistry, 2017, 234(1):285-294.
[34] DONG S X, YANG X S, ZHAO L, et al.Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd.husks against foodborne pathogenic bacteria[J].Industrial Crops & Products, 2020, 149:112 350.
[35] LETELIER M E, ROJAS C R, JOFRE S S, et al.Surfactant and antioxidant properties of an extract from Chenopodium quinoa Willd seed coats[J].Journal of Cereal Science, 2011, 53(2):239-243.
[36] NSIMBA R Y, KIKUZAKI H, KONISHI Y.Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp seeds[J].Food Chemistry, 2007, 106(2):760-766.
[37] 杜静婷, 陈超, 范三红.响应面法优化藜麦糠皂苷的提取及抗氧化活性[J].山西农业科学, 2016, 44(7):932-937.
DU J T, CHEN C, FAN S H.Optimization of extraction conditions for saponins from Chenopodium quinoa bran by response surface[J].Journal of Shanxi Agricultural Sciences, 2016, 44(7):932-937.
[38] STUARDO M, MARTIN R S.Antifungal properties of quinoa (Chenopodium quinoa Willd) alkali treated saponins against Botrytis cinerea[J].Industrial Crops & Products, 2007, 27(3):296-302.
[39] SUN X Y, YANG X S, XUE P, et al.Improved ant ibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria[J].Bmc Complementary & Alternative Medicine, 2019, 19(1):46.
[40] YAO Y, YANG X S, SHI Z X, et al.Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 Macrophages Cells[J].Journal of Food Science, 2014, 79(5);1 018-1 023.
[41] YANG Y, LAVAL S, YUB B.Chemical synthesis of saponins[J].Advances in Carbohydrate Chemistry and Biochemistry,2014, 71:137-226.
[42] SUN Y X, LI M Q, LIU J C.Haemolytic activities and adjuvant effect of Anemone raddeana saponins (ARS) on the immune responses to ovalbumin in mice[J].International Immunopharmacology, 2008, 8(8):1 095-1 102.
[43] SUN H X, XIE Y, YE Y P.Advances in saponin-based adjuvants[J].Vaccine, 2009, 27(12):1 787-1 796.
[44] SONG X M, HU S H.Adjuvant activities of saponins from traditional Chinese medicinal herbs[J].Vaccine, 2009, 27(36):4 883-4 890.
[45] RAVINDRA C J, RICARDO S M, NAVARRETE C S, et al.Efficacy of quinoa (Chenopodium quinoa) saponins against golden apple snail (Pomacea canaliculata) in the Philippines under laboratory conditions[J].Crop Protection, 2007, 27(3):553-557.
[46] MARTIN R S, NDJOKO K, HOSTETTMAN K.Novel molluscicide against Pomacea canaliculata based on quinoa (Chenopodium quinoa) saponin[J].Crop Protection, 2007, 27(3):310-319.
[47] RUIZ M C, JONES H C, SCHLOTTERBECK T, et al.Safety and efficacy of quinoa (Chenopodium quinoa) saponins derived molluscicide to control of Pomacea maculata in rice fields in the Ebro Delta, Spain[J].Crop Protection, 2018, 111:42-49.
[48] YAO Y, ZHU Y Y, GAO Y, et al.Suppressive effects of saponin-enriched extracts from quinoa on 3T3-L1 adipocyte differentiation[J].Food & Function, 2015, 6(10):3 282-3 290.
[49] FOUCAULT A S, EVEN P, LAFONT R, et al.Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet[J].Phys Behavior, 2014, 128:226-231.
[50] 冯焕琴. 藜麦活性物质提取及测定方法的比较[D].兰州:甘肃农业大学, 2017.
FENG H Q.Comparison of extraction and determination methods of active substances in quinoa[D].Lanzhou:Gansu Agricultural University, 2017.