 A-VILLALBA等[24]方法将发酵液与50%酸性乙醇(体积比1∶1)涡旋混匀,40 W超声20 min,过0.2 μm微孔滤膜,安捷伦DB-FFAP(30 m×0.53 mm×0.5 μm)柱,进样量l μL,气相色谱仪测定发酵液中乙酸、丙酸、正丁酸、异丁酸、戊酸、异戊酸含量。pH(pH仪)、总糖含量(苯酚-硫酸法)、还原糖含量(3,5-二硝基水杨酸(DNS法)、菌落总数(平板菌落计数法)。
A-VILLALBA等[24]方法将发酵液与50%酸性乙醇(体积比1∶1)涡旋混匀,40 W超声20 min,过0.2 μm微孔滤膜,安捷伦DB-FFAP(30 m×0.53 mm×0.5 μm)柱,进样量l μL,气相色谱仪测定发酵液中乙酸、丙酸、正丁酸、异丁酸、戊酸、异戊酸含量。pH(pH仪)、总糖含量(苯酚-硫酸法)、还原糖含量(3,5-二硝基水杨酸(DNS法)、菌落总数(平板菌落计数法)。近年来,胃肠道菌群常作为独立实体来研究[1]。随着生物信息学工具发展,人们越来越意识到胃微生物群的多样性和复杂性[2]以及对疾病的潜在诱导作用[3]。大肠与小肠微生物的种类与分布明显不同,且不同肠段所富集微生物的代谢通路也不同[4-5]。胃肠道的稳态依赖于健康的胃肠道菌群[6],其比例、数量及稳定状态与多种营养相关疾病密切相关[7-8],同时参与机体食物消化、营养代谢吸收、免疫调节、肠道稳态的维持等重要生理过程[9-10]。肠道微生物体外模拟发酵可避免机体对代谢产物的吸收,直接反映微生物的代谢状况[11]。英国LAWLEY团队证实体外培养的细菌菌落与原始样品存在72%相同的基因组序列,表明肠道细菌可以在体外培养、存活[12]。YIN等[13]研究表明,YCFA培养基能够在体外肠道微生物模拟系统中模拟人肠道微生物的组成,相似率达70%以上。
膳食纤维是肠道菌群结构和功能调控者,对肠道菌群起重要调节作用[14-16]。魔芋葡甘聚糖(konjac glucomannan,KGM)是纯天然高分子膳食纤维,是一种水溶性高黏度多糖[17]。试验证实KGM具有改善结肠微生物多样性、润肠通便、调节脂质代谢等功能[18-19]。魔芋葡甘低聚糖(konjac oligosaccharides,KOS)是以KGM为原料,经物理、化学、生物法降解而得,研究表明KOS同样具有良好肠道益生性、降血糖等作用[20]。迄今为止,应用膳食纤维调节肠道菌群主要集中在粪便及结肠内容物上,而同一个体不同部位微环境的变化未见报道。本研究分别采集小鼠胃、小肠、大肠中微生物,通过体外模拟发酵探究KGM和KOS与不同胃肠段微环境的影响,为应用KGM和KOS改善胃肠道健康提供理论依据。
魔芋葡甘聚糖,由重庆康家客食品有限公司提供。魔芋葡甘低聚糖,采用课题组方法自制[21]。
1.2.1 内容物样本采集及胃肠微生物发酵液制备
无菌条件采集新鲜健康成年昆明种小鼠胃、小肠(空回肠)、大肠(盲结肠)内容物。与经灭菌、厌氧处理的生理盐水(质量分数10%)涡旋振荡3 min,滤液即为内容物样品。参考朱立颖[11]和ZE等[22]方法,配制含7 g/L KGM和KOS(干基)-YCFA基础发酵培养基,121 ℃高压灭菌15 min,备用。以葡萄糖(glucose,G)和不加碳源的培养基(control,C)为对照。按体积浓度10%的接种量将内容物样品接种至培养基中,37℃,80 r/min摇床振荡,获得胃肠微生物发酵液。分别于0、1、2、3 h采集胃发酵液,0、2、4、6、8 h采集小肠发酵液,0、3、6、9、12 h采集大肠发酵液。
1.2.2 发酵液相关指标测定
参考FABEK等[23]方法采用流变仪,60 mm、4°锥板夹具,37 ℃,比较200 s-1剪切速率下不同发酵液表观黏度。参考GARC A-VILLALBA等[24]方法将发酵液与50%酸性乙醇(体积比1∶1)涡旋混匀,40 W超声20 min,过0.2 μm微孔滤膜,安捷伦DB-FFAP(30 m×0.53 mm×0.5 μm)柱,进样量l μL,气相色谱仪测定发酵液中乙酸、丙酸、正丁酸、异丁酸、戊酸、异戊酸含量。pH(pH仪)、总糖含量(苯酚-硫酸法)、还原糖含量(3,5-二硝基水杨酸(DNS法)、菌落总数(平板菌落计数法)。
A-VILLALBA等[24]方法将发酵液与50%酸性乙醇(体积比1∶1)涡旋混匀,40 W超声20 min,过0.2 μm微孔滤膜,安捷伦DB-FFAP(30 m×0.53 mm×0.5 μm)柱,进样量l μL,气相色谱仪测定发酵液中乙酸、丙酸、正丁酸、异丁酸、戊酸、异戊酸含量。pH(pH仪)、总糖含量(苯酚-硫酸法)、还原糖含量(3,5-二硝基水杨酸(DNS法)、菌落总数(平板菌落计数法)。
1.2.3 数据分析
每组试验进行3次重复,试验结果以平均值±标准差表示。采用SPSS 20.0软件进行差异显著性分析, Sigmaplot 10.0软件用于图形绘制。
食物在胃部2~3 h就排空,小肠吸收面积大,食物可存留3~8 h,大肠停留12 h左右[25]会形成粪便。随着发酵时间的延长,不同胃肠段的不同处理组pH均显著降低(P<0.05),空白组降低幅度小(图1)。初始pH为胃<小肠<大肠,KOS的初始pH较其余分组低,由于KOS是用pH 6.0柠檬酸-柠檬酸钠缓冲液制备而成[21]。KGM组pH胃6.82(0 h)至6.78(3 h),小肠6.83(0 h)至6.42(8 h),大肠6.90(0 h)至5.70(12 h)。KOS组pH胃6.77(0 h)至6.72(3 h),小肠6.78(0 h)至6.23(8 h),大肠6.83(0 h)至4.88(12 h)。试验结果表明微生物发酵的代谢产物会改变发酵液的pH。
短链脂肪酸(short-chain fatty acids,SCFAs)是膳食纤维细菌发酵的主要代谢产物[26]。由表1可以看出,随着发酵时间的延长,胃发酵液中只有乙酸含量增加,小肠和大肠发酵液中SCFAs均有不同程度的增加。各受拭物组发酵液SCFAs含量增加幅度显著高于空白组。KGM组胃SCFAs总量由1.437 16E-02(0 h)增加到1.724 70E-02(3 h),小肠由1.504 03E-02(0 h)增加到8.385 17E-02(8 h),大肠由2.888 50E-02(0 h)增加到1.798 89E-01(12 h)。KOS不同发酵液的变化趋势与KGM相同。肠道可迅速吸收SCFAs[27],从而调节机体健康[28-29]。本研究结果表明,KGM和KOS可促进胃肠道微生物发酵产生SCFAs。

A-胃;B-小肠;C-大肠
图1 不同发酵液pH变化
Fig.1 Different fermentation broth pH changes
注:不同小写字母表示同一分组不同发酵时间0.05水平有显著差异(P<0.05)(下同)
表1 不同发酵液短链脂肪酸含量 单位:μg/mL
Table 1 Short-chain fatty acids in different fermentation broth

分组时间/h乙酸丙酸异丁酸丁酸异戊酸戊酸总SCFAs胃对照GKGMKOS01.604 24E-02±2.944 89E-03b-----1.604 24E-02±2.944 89E-03b11.634 55E-02±8.087 99E-05ab-----1.634 55E-02±8.087 99E-05ab21.643 55E-02±2.471 30E-05ab-----1.643 55E-02±2.471 30E-05ab31.649 77E-02±2.435 96E-05a-----1.649 77E-02±2.435 96E-05a01.903 46E-02±1.423 11E-04d-----1.903 46E-021±42 311E-04d11.938 86E-02±2.149 70E-05c-----1.938 86E-02±2.149 70E-05c21.966 21E-02±3.611 46E-05b-----1.966 21E-02±3.611 46E-05b32.077 34E-02±8.197 81E-05a-----2.077 34E-02±8.197 81E-05a01.437 16E-02±6.842 12E-05d-----1.437 16E-02±6.842 12E-05d11.469 57E-02±9.759 41E-06c-----1.469 57E-02±9.759 41E-06c21.590 16E-02±4.203 45E-05b-----1.590 16E-02±4.203 45E-05b31.724 70E-02±5.100 77E-05a-----1.724 70E-02±5.100 77E-05a01.624 28E-02±2.744 46E-04d-----1.624 28E-02±2.744 46E-04d12.020 89E-02±3.982 82E-04c-----2.020 89E-02±3.982 82E-04c22.204 64E-02±6.158 14E-04b-----2.204 64E-02±6.158 14E-04b32.355 16E-02±5.037 78E-04a-----2.355 16E-02±5.037 78E-04a
续表1

分组时间/h乙酸丙酸异丁酸丁酸异戊酸戊酸总SCFAs小肠对照GKGMKOS01.410 70E-02±9.959 20E-05e--3.636 68E-03±8.034 01E-05e--1.774 37E-02±1.779 10E-04e21.559 59E-02±1.920 18E-05d--4.197 01E-03±7.894 66E-05d--1.979 30E-02±9.799 72E-05d41.906 96E-02±4.782 37E-05c--5.285 26E-03±5.665 66E-05c2.067 41E-03±3.141 93E-05c-2.642 23E-02±6.909 04E-05c63.458 64E-02±6.923 36E-04b--6.276 13E-03±6.448 85E-05b2.783 73E-03±4.980 66E-05b-4.364 62E-02±6.496 42E-04b85.703 48E-02±3.398 92E-04a-1.631 13E-03±2.261 72E-06a6.526 30E-03±6.449 17E-05a3.187 32E-03±8.329 91E-05a-6.837 95E-02±4.863 61E-04a01.070 39E-02±6.576 73E-06d--7.131 86E-03±6.336 17E-05e-1.031 56E-03±1.611 96E-06e1.886 74E-02±6.878 53E-05e21.188 67E-02±1.121 73E-04d-1.714 31E-03±4.102 96E-06d7.203 12E-03±7.598 48E-05d1.639 52E-03±6.457 65E-07d1.428 02E-03±5.324 68E-05d2.387 17E-02±7.901 84E-05d43.295 09E-02±1.396 95E-04c-1.993 58E-03±2.421 96E-05c7.363 16E-03±3.305 55E-05c2.113 53E-03±1.266 52E-05c1.735 47E-03±9.821 64E-06c4.615 66E-02±1.604 07E-04c63.966 54E-02±5.877 40E-04b-2.385 65E-03±2.161 86E-05b7.433 54E-03±5.266 36E-05b2.777 13E-03±6.905 61E-04b1.754 21E-03±7.273 61E-06b5.401 59E-02±2.457 09E-04b87.138 41E-02±1.090 37E-03a-2.491 20E-03±1.640 43E-05a7.905 99E-03±1.942 15E-05a2.796 74E-03±1.626 40E-05a1.889 29E-03±1.914 80E-05a8.646 73E-02±1.042 85E-03a08.753 57E-03±1.227 76E-04e-2.029 32E-03±4.734 37E-06e4.257 44E-03±2.226 00E-04e--1.504 03E-02±1.977 99E-04e21.122 51E-02±2.406 31E-04d-2.665 24E-03±8.910 53E-06d5.417 20E-03±5.705 44E-05d1.239 42E-03±1.182 43E-06d-2.054 70E-02±1.782 01E-04d42.352 95E-02±4.761 37E-04c-2.993 01E-03±3.373 07E-05c5.856 73E-03±5.260 08E-05c2.368 20E-03±4.508 17E-05c-3.478 08E-02±5.560 98E-04c65.133 52E-02±5.576 05E-04b-3.293 98E-03±1.622 49E-05b5.879 46E-03±2.800 54E-06b3.022 26E-03±3.937 93E-05b-6.353 09E-02±5.387 81E-04b87.015 67E-02±2.442 45E-04a-3.664 66E-03±4.599 16E-05a6.311 12E-03±4.255 73E-04a3.719 26E-03±1.718 00E-05a-8.385 17E-02±2.060 26E-04a02.122 96E-02±2.361 85E-04e-7.736 12E-04±3.183 23E-06e5.867 64E-03±3.922 63E-05e--2.787 09E-02±2.515 70E-04e22.307 53E-02±6.214 08E-05d-7.763 03E-04±1.453 50E-04d6.224 85E-03±2.215 63E-05d1.233 94E-03±1.317 77E-05d-3.131 04E-02±1.337 73E-04d43.096 66E-02±4.843 80E-04c-9.824 39E-04±5.240 69E-06c6.511 43E-03±1.015 87E-05c1.408 81E-03±4.156 22E-06c-3.986 93E-02±5.033 38E-04c65.019 99E-02±1.820 83E-04b-1.020 22E-03±3.327 68E-07b7.591 31E-03±1.622 26E-05b1.543 24E-03±4.557 76E-06b-6.035 46E-02±1.732 16E-04b87.394 94E-02±7.387 95E-05a-1.285 98E-03±1.305 86E-04a7.799 86E-03±2.361 11E-06a1.601 71E-03±1.698 33E-06a-8.463 69E-02±1.497 28E-04a
续表1

分组时间/h乙酸丙酸异丁酸丁酸异戊酸戊酸总SCFAs大肠对照GKGMKOS01.838 53E-02±4.656 95E-05e--6.262 00E-03±3.997 55E-05e--2.464 73E-02±2.035 85E-05e31.917 74E-02±3.965 38E-05d--6.408 60E-03±4.742 64E-05d--2.558 60E-02±5.935 87E-05d63.796 35E-02±1.703 75E-04c--6.529 33E-03±2.158 19E-05c--4.449 28E-02±1.804 73E-04c95.281 19E-02±3.772 39E-04b1.665 79E-03±1.508 21E-05b-6.966 95E-03±8.751 59E-05b--6.144 46E-02±4.515 15E-04b126.365 09E-02±4.557 47E-04a1.762 34E-03±2.324 06E-05a-7.239 78E-03±1.242 97E-04a--7.265 30E-02±5.910 44E-04a02.290 42E-02±5.218 32E-04e2.503 02E-03±3.948 85E-06e-5.518 25E-03±5.875 63E-05e--3.092 54E-02±5.363 17E-04e32.599 25E-02±2.341 90E-04d2.596 86E-03±1.999 53E-05d3.690 51E-03±2.195 05E-05d6.467 80E-03±6.836 80E-05d--3.874 77E-02±1.468 82E-04d64.499 12E-02±5.447 17E-04c2.998 58E-03±1.167 17E-05c4.168 03E-03±5.271 32E-05c6.735 18E-03±4.271 92E-05c--5.889 30E-02±5.610 42E-04c96.847 25E-02±4.813 39E-04b3.196 49E-03±1.398 16E-04b5.310 02E-03±1.645 45E-05b7.924 31E-03±3.533 38E-05b--8.490 33E-02±5.266 53E-04b128.053 15E-02±6.397 99E-04a3.693 92E-03±8.129 90E-06a6.042 75E-03±4.004 37E-05a8.502 07E-03±6.286 81E-04a--9.877 02E-02±1.099 34E-04a01.738 11E-02±5.010 46E-05e2.795 28E-03±1.869 91E-05e3.070 23E-03±4.596 67E-05e5.638 40E-03±4.595 36E-05e--2.888 50E-02±6.671 34E-05e31.948 15E-02±1.627 67E-04d6.000 64E-03±1.917 94E-05d3.625 24E-03±2.658 22E-05d5.852 65E-03±1.687 72E-05d--3.496 00E-02±2.171 57E-04d65.203 60E-02±5.715 12E-04c7.595 20E-03±6.956 10E-05c4.335 22E-03±4.328 74E-06c5.918 04E-03±6.314 73E-05c--6.988 44E-02±5.855 62E-04c91.333 68E-01±1.054 49E-03b1.651 54E-02±5.484 49E-05b4.570 96E-03±7.801 75E-06b6.071 90E-03±4.432 80E-05b--1.605 26E-01±1.063 79E-03b121.514 29E-01±7.897 95E-04a1.765 52E-02±5.453 74E-05a4.505 78E-03±3.526 48E-04a6.298 39E-03±8.324 53E-06a--1.798 89E-01±1.014 45E-03a02.519 74E-02±7.488 88E-06e3.128 20E-03±7.177 63E-06e-5.844 62E-03±5.802 41E-06e--3.417 02E-02±8.223 31E-06e33.077 70E-02±3.060 50E-04d4.340 39E-03±1.341 38E-05d1.905 98E-03±5.58 903E-06d5.927 40E-03±3.409 17E-06d--4.295 08E-02±3.188 12E-04d65.996 66E-02±2.056 92E-04c8.006 97E-03±1.337 70E-05c2.005 25E-03±6.451 94E-06c6.515 54E-03±1.517 56E-05c--7.649 44E-02±2.061 56E-04c91.902 32E-01±2.423 58E-04b1.269 88E-02±6.351 64E-06b2.940 29E-03±1.263 58E-06b6.735 13E-03±8.118 17E-06b--2.126 07E-01±2.441 27E-04b122.358 68E-01±3.666 60E-03a1.367 40E-02±4.227 96E-05a3.339 08E-03±4.359 58E-04a6.817 29E-03±9.185 83E-06a--2.596 99E-01±3.511 18E-03a
注:表中“-”表示未检出,不同小写字母代表差异显著(P<0.05)(下同)
胃肠道微生物是宿主不可缺少的一部分,在机体健康中扮演重要角色[30-31]。在门和属水平上,大肠间(盲肠和结肠)相似度大于小肠间(十二指肠、空肠、回肠)相似度[32-33]。固本试验采集整个胃内容物,小肠空回肠部,大肠盲结肠部作为体外发酵微生物来源。随着发酵时间的延长,菌落总数均呈上升的趋势,对照组上升幅度小,其余受拭物组上升幅度大(图2)。不同胃肠段菌落总数:大肠>小肠>胃。KGM组胃菌落总数由1.63E+04(0 h)上升到8.57E+04(3 h),小肠由3.20E+05(0 h)上升到1.93E+07(8 h),大肠由6.23E+05(0 h)上升到5.50E+08(12 h)。KOS组不同发酵液菌落总数的大小变化与KGM基本一致。同一个体因不同胃肠段pH、胃肠蠕动强度等生理状态不同,微生物数量沿消化道(胃→小肠→大肠)自上而下逐渐增多[34-35]。本试验菌落总数的结果同样表明KGM和KOS显著增加发酵液中微生物的数量,且胃<小肠<大肠。

A-胃;B-小肠;C-大肠
图2 不同发酵液菌落总数
Fig.2 Total colonies number in different fermentation broth
食用黏性膳食纤维可对机体产生多种有益功能[36-38]。随发酵时间延长,除KGM组,其余各个处理组发酵液的黏度无显著变化(P>0.05)(表2)。KGM组发酵液的黏度明显高于其余各组,大肠发酵9 h后发酵液黏度由116.116 7 mPa·s显著降低至37.245 mPa·s(P<0.05),发酵12 h后发酵液黏度有所增加。本试验中KGM显著增加发酵液黏度,表明KGM可对机体产生有益功能。
表2 不同发酵液黏度 单位:mPa·s
Table 2 Viscosity of different fermentation broth

时间/h胃对照GKGMKOS00.947 6±0.226 7a0.826 1±0.003 8b291.750 0±8.407 1b0.832 8±0.004 7b10.824 7±0.005 2b0.798 8±0.017 0b257.023 3±6.288 9c0.817 1±0.009 1b20.894 8±0.037 3b0.811 6±0.016 9b277.736 7±4.331 0bc0.880 4±0.050 5ab30.963±10.188 9a1.054 4±0.046 2a547.983 3±17.012 3a0.949 0±0.019 2a时间/h小肠对照GKGMKOS00.808 9±0.008 0a0.800 1±0.009 6a768.890 0±71.135 8a0.867 9±0.015 4a20.765 0±0.006 1b0.793 9±0.003 6a412.853 3±28.555 7b0.813 5±0.002 8b40.803 9±0.015 2a0.802 3±0.007 3a368.296 7±20.926 9c0.813 1±0.002 3b60.785 9±0.003 2ab0.786 4±0.020 5a208.280 0±16.085 7c0.809 9±0.001 4b80.802 8±0.011 4a0.800 6±0.002 5a324.173 3±3.146 6c0.799 0±0.006 4b时间/h大肠对照GKGMKOS01.973 5±0.525 5b0.996 5±0.067 7a116.116 7±9.272 2a1.663 8±0.326 2b31.326 1±0.188 4b1.004 1±0.090 1a104.186 7±1.816 9a3.714 2±0.615 4a61.023 0±0.065 1b0.894 4±0.055 1ab57.456 7±3.967 0b2.984 5±0.425 8a91.718 0±0.095 1b0.901 9±0.051 9ab37.245 3±0.986 5c1.083 9±0.111 1b122.108 6±0.077 5a0.824 6±0.020 8b60.113 7±3.410 0b1.818 4±0.014 7b
多糖等大分子膳食纤维常作为微生物生长繁殖的碳源和能源物质[39-40]。随发酵时间延长,各发酵液中总糖含量均显著降低(P<0.05)(图3)。含KGM胃发酵液从139.511 μg/mL(0 h)降低到134.879 μg/mL(3 h),小肠发酵液从261.329 μg/mL(0 h)降低到189.900 μg/mL(8 h),大肠发酵液从173.537 μg/mL(0 h)降低到120.203 μg/mL(12 h),KOS有同样降低趋势。表明KGM和KOS均会被不同胃肠段微生物发生不同程度的消耗,导致发酵液中总糖含量降低。
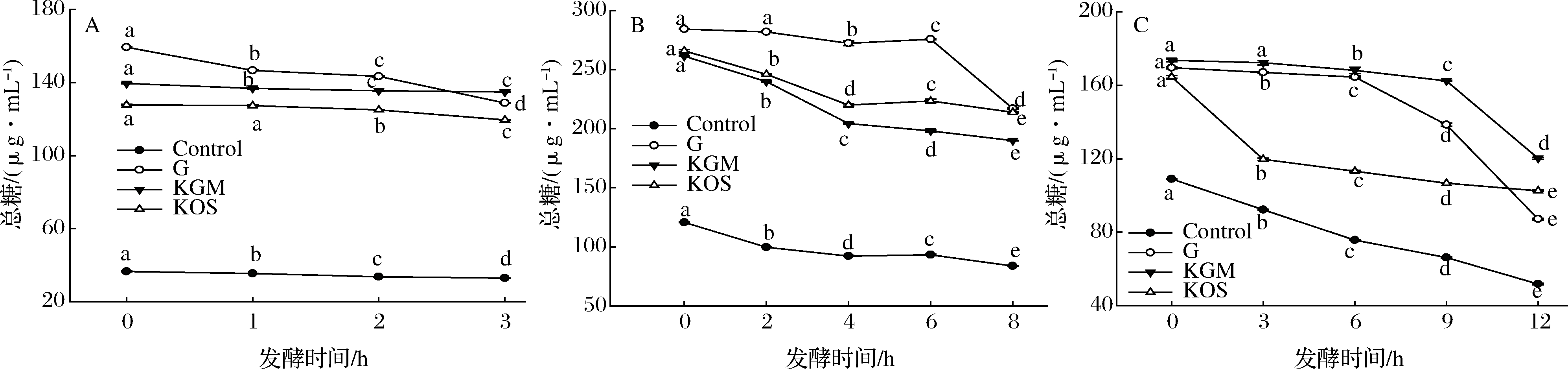
A-胃;B-小肠;C-大肠
图3 不同发酵液总糖含量
Fig.3 Total sugar contents in different fermentation broth
还原糖是微生物代谢过程中优先利用的碳源[41],除KGM组,其余各组还原糖含量随着发酵时间的延长不断降低(图4)。大肠发酵液中KGM组还原糖含量由0.399 mg/mL(0 h)增加到0.441 mg/mL(6 h)再降低至0.381 mg/mL(12 h),KOS组由2.778 mg/mL(0 h)降低至1.592 mg/mL(12 h),G组由4.564 mg/mL(0 h)降低至2.679 mg/mL(12 h)。主要是因KGM的糖苷键会被微生物破环导致体系中还原糖含量的增加,该结果与HUANG等[42],GAO等[43]探究多糖发酵液中总糖和还原糖含量变化趋势一致。

A-胃;B-小肠;C-大肠
图4 不同发酵液还原糖含量
Fig.4 Reducing sugar contents in different fermentation broth
不同胃肠段微生物可利用KGM和KOS来降低发酵液pH,增加SCFAs含量,增加发酵液中微生物菌落总数。同时KGM显著增加胃及小肠发酵液黏度。表明KGM和KOS可改善不同胃肠段微环境向有利于机体健康的方向发展。本研究为KGM和KOS的应用提供新的思路,为后续探究KGM和KOS与不同胃肠段微生物的具体作用机制提供理论依据。
[1] WATT E, GEMMELL M R, BERRY S, et al. Extending colonic mucosal microbiome analysis-assessment of colonic lavage as a proxy for endoscopic colonic biopsies[J]. Microbiome, 2016, 4(1): 61-76.
[2] HUNT R H, YAGHOOBI M. The esophageal and gastric microbiome in health and disease[J]. Gastroenterol Clin North Am, 2017, 46(1): 121-141.
[3] HE C, YANG Z, LU N. Imbalance of gastrointestinal microbiota in the pathogenesis of helicobacter pylori-associated diseases[J]. Helicobacter, 2016, 21(5): 337-348.
[4] CHANG E B, MARTINEZ-GURYN K. Small intestinal microbiota: The neglected stepchild needed for fat digestion and absorption[J]. Gut Microbes, 2019, 10(2): 235-240.
[5] KASTL A J, TERRY N A, ALBENBERG L G, et al. The structure and function of the human small intestinal microbiota: current understanding and future directions[J]. Cellular and Molecular Gastroenterology and Hepatology, 2020,9(1): 1-13.
[6] MARCHRSI J R, ADAMS D H, FAVA F, et al. The gut microbiota and host health: A new clinical frontier[J]. Gut, 2016, 65(2): 330-339.
[7] BOULANGE C L, NEVES A L, CHILLOUX J, et al. Impact of the gut microbiota on inflammation, obesity, and metabolic disease[J]. Genome Medicine, 2016, 8(1): 42-54.
[8] YUICHIRO Y. Gut microbiota in health and disease[J]. Annals of Nutrition & Metabolism, 2017,71(3-4):242-246.
[9] VUIK F E R, DICKSVED J, LAM S Y, et al. Composition of the mucosa-associated microbiota along the entire gastrointestinal tract of human individuals[J]. United European Gastroenterology Journal, 2019, 7(7): 897-907.
[10] WLODARSKA M, KOSTIC A D, XAVIER R J. An integrative view of microbiome-host interactions in inflammatory bowel diseases[J]. Cell Host & Microbe, 2015, 17(5): 577-591.
[11] 朱立颖. 一种肠道微生物体外模拟培养方法: 中国, CN201711069219.7[P]. 2017-11-03.
[12] BROWNE H P, FORSTER S C, ANONYE B O, et al. Culturing of 'unculturable' human microbiota reveals novel taxa and extensive sporulation[J]. Nature, 2016,533(7 604): 543-546.
[13] YIN Y, LEI F, ZHU L, et al. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression[J]. The ISME Journal, 2010,4(3):367-376.
[14] RAJOA M S R, SHI J, MEHWISH H M, et al. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health[J]. Food Science and Human Wellness, 2017, 6(3): 121-130.
[15] DESAI M S, SEEKATZA M, KOROPATKIN N M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility[J]. Cell, 2016, 167(5): 1 339-1 353.
[16] ZOU J, CHASSAING B, SINGH V, et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring il-22-mediated colonic health[J]. Cell Host Microbe, 2018,23(1):41-53.
[17] WU C, LI Y, SUN J, et al. Novel konjacglucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging[J]. Food Hydrocolloids, 2020,98:105 245.
[18] BEHERA S S, RAY R C. Nutritional and potential health benefits of konjac glucomannan, a promising polysaccharide of elephant foot yam, Amorphophallus konjac K. Koch: A review[J]. Food Reviews International, 2017,33(1):22-43.
[19] TESTER R F, Al-GHAZZEWI F H. Beneficial health characteristics of native and hydrolysedkonjac (Amorphophallus konjac) glucomannan[J]. J Sci Food Agric, 2016,96(10):3 283-3 291.
[20] 秦清娟. 魔芋葡甘低聚糖毒理学性质及肠道益生性研究[D]. 重庆:西南大学, 2015.
[21] 张迅. 珠芽魔芋葡甘低聚糖制备、性质及对AFB_1吸附能力的研究[D]. 重庆:西南大学, 2018.
[22] ZE X, DUNCAN S H, LOUIS P, et al. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon[J]. The ISME journal, 2012,6(8):1 535-1 543.
[23] FABEK H, MESSERSCHMIDT S, BRULPORT V, et al. The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion[J]. Food Hydrocolloids, 2014,35:718-726.
[24] GARC A-VILLALBA R, GIM
A-VILLALBA R, GIM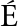 NEZ-BASTIDA J A, GARC
NEZ-BASTIDA J A, GARC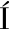 A-CONESA M T, et al. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples[J]. J Sep Sci, 2012,35(15):1 906-1 913.
A-CONESA M T, et al. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples[J]. J Sep Sci, 2012,35(15):1 906-1 913.
[25] BORNHORST G M, GOUSETI O, WICKHAM M S J,et al. Engineering digestion: Multiscale processes of food digestion[J]. Journal of Food Science,2016,81(3):R534-R543.
[26] DALILE B, VAN OUDENHOVE L, VERVLIET B, et al. The role of short-chain fatty acids in microbiota-gut-brain communication[J]. Nat Rev Gastroenterol Hepatol, 2019,16(8):461-478.
[27] KABAT A M, SRINIVASAN N, MALOY K J. Modulation of immune development and function by intestinal microbiota[J]. Trends in Immunology, 2014,35(11):507-517.
[28] HAN X, SONG H, WANG Y, et al. Sodium butyrate protects the intestinal barrier function in peritonitic mice[J]. International Journal of Clinical and Experimental Medicine, 2015,8(3):4 000-4 007.
[29] RIOS-COVI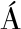 N D, RUAS-MADIEDO P, MARGOLLES A, et al. Intestinal short chain fatty acids and their link with diet and human health[J]. Frontiers in Microbiology, 2016,7:185-187.
N D, RUAS-MADIEDO P, MARGOLLES A, et al. Intestinal short chain fatty acids and their link with diet and human health[J]. Frontiers in Microbiology, 2016,7:185-187.
[30] TREMAROLI V, B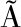 CKHED F. Functional interactions between the gut microbiota and host metabolism[J]. Nature, 2012,489(7415):242-249.
CKHED F. Functional interactions between the gut microbiota and host metabolism[J]. Nature, 2012,489(7415):242-249.
[31] D′ ARGENIO V, SALVATORE F. The role of the gut microbiome in the healthy adult status[J]. Clinica Chimica Acta, 2015,451(Pt A):97-102.
[32] GU S, CHEN D, ZHANG J N, et al. Bacterial community mapping of the mouse gastrointestinal tract[J]. PLoS One, 2013,8(10):1-9.
[33] COSTELLO E K, LAUBER C L, HAMADY M, et al. Bacterial community variation in human body habitats across space and time[J]. Science, 2009,326(5 960):1 694-1 697.
[34] QIN J, LI R, RAES J, et al. A human gut microbial gene catalogue established by metagenomic sequencing[J]. Nature, 2010,464(7 285):59-65.
[35] DELGADO S, CABRERA-RUBIO R, MIRA A, et al. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods[J]. Microb Ecol, 2013,65(3):763-772.
[36] GOEL A. Review: Viscous fiber reduces HbA1c, fasting glucose, and insulin resistance in type 2 diabetes[J]. Ann Intern Med, 2019,170(10):JC51.
[37] ISLAM A, CIVITARESE A E, HESSLINK R L, et al. Viscous dietary fiber reduces adiposity and plasma leptin and increases muscle expression of fat oxidation genes in rats[J]. Obesity,2012,20(2):349-355.
[38] PANDE S, PLATEL K, SRINIVASAN K. Anti-hypercholesterolaemic influence of dietary tender cluster beans (Cyamopsis tetragonoloba) in cholesterol fed rats[J]. Indian J Med Res, 2012,135(3):401-406.
[39] SINGH J, METRANI R, SHIWANAGOUDRA S R, et al. Review on bile acids: effects of the gut microbiome, interactions with dietary fiber, and alterations in the bioaccessibility of bioactive compounds[J]. J Agric Food Chem, 2019,67(33):9 124-9 138.
[40] ZHAO J, LIU P, WU Y, et al. Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets[J]. Journal of Agricultural and Food Chemistry, 2018,66(30):7 995-8 004.
[41] TIAN S, WANG J, YU H, et al. Changes in Ileal microbial composition and microbial metabolism by an early-life galacto-oligosaccharides intervention in a neonatal porcine model[J]. Nutrients, 2019,11(8):1 753-1 756.
[42] HUANG F, LIU L, ZHANG R, et al. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide[J]. International Journal of Biological Macromolecules, 2019,140:965-972.
[43] GAO J, LIN L, CHEN Z, et al. In vitro digestion and fermentation of three polysaccharide fractions from laminaria japonica and their impact on lipid metabolism-associated human gut microbiota[J]. Journal of Agricultural and Food chemistry, 2019,67(26):7 496-7 505.