黄酮类化合物是一类重要的天然产物,种类众多,广泛存在于蕨类和高等植物中,具有抗肿瘤、抗菌抗病毒、抗自由基等生物活性。此外,对人体还有降血压血脂、抗衰老、提高机体免疫力等活性[1]。黄酮类化合物在新药研制、食品贮藏、化妆品工业等领域均有重要应用,但其在植物中含量低且不易提取,基于此,对黄酮类化合物人工合成和加工修饰显得极为重要。
近年,随着对黄酮类化合物植物合成相关酶及其基因的研究不断开展,微生物基因工程合成黄酮类化合物也取得了较大进展。同时,微生物修饰黄酮类化合物也有不少报道,自2000年以来,已发现多种可转化黄酮类化合物的微生物,部分微生物还可通过自身代谢直接合成黄酮类化合物[2-3]。另外,化学方法合成黄酮类化合物及结构修饰也有很多报道[4-7]。不论是微生物合成及转化,还是化学方法的合成和结构修饰,近年均无全面阐述,笔者综述近年国内外黄酮类化合物合成及结构修饰相关研究,旨在为黄酮类化合物的开发利用提供参考。
1 黄酮类化合物分类及生物合成途径
1.1 黄酮类化合物分类
黄酮类化合物基本骨架为C6-C3-C6结构,中间C3结构可以是开链,或者与苯环一起聚合成氧杂环, C3链是否成环及其氧化程度可作为黄酮类化合物的分类依据之一。此外,根据B环与C环的链接位置不同,C环是否含酮基,以及主体结构上取代基的位置和数目不同等特征,黄酮类化合物可分为不同的种类。目前发现并鉴定的黄酮类化合物已超过一万种[8],主要有黄酮类、黄酮醇类、异黄酮类、黄烷酮类、查尔酮类和花色素等。其存在形式有结合态和游离苷元两种,大多与葡萄糖、棉子糖、半乳糖、鼠李糖等以氧糖苷成结合态,其中以葡萄糖最为普遍。其分类及物质举例如表1所示。
1.2 黄酮类化合物生物合成途径
在植物次生代谢中,黄酮类化合物的代谢研究最为深入和清晰,其合成酶涉及苯丙氨酸解氨酶(phenylalanine ammonia-lyase, PAL)、酪氨酸解氨酶(tyrosineammonialyase, TAL)、肉桂酸4-羟化酶 (cinnamic acid-4-hydroxylase, C4H)、香豆酰-CoA连接酶(4-coumaryl-CoA ligase, 4CL)、查尔酮合酶(chalcone synthase, CHS)、查尔酮异构酶(chalcone isomerase, CHI)、黄酮合酶(flavonol synthase, FNS)、异黄酮合酶(isoflavone synthase,IFS)、类黄酮3′羟化酶(flavonoid 3′-hydroxylase, F3′H)等。
黄酮类化合物生物合成途径涉及醋酸盐途径(A环合成)和莽草酸盐途径(B环和中间C3合成),A环由葡萄糖转化生成的3个丙二酰-CoA分子合成,B环由苯丙氨酸生成的4-香豆酰-CoA通过莽草酸途径合成[9]。A环和B环在CHS催化下缩合成查耳酮,查耳酮再经CHI催化生成二氢黄酮。二氢黄酮是大部分黄酮类化合物的共同前体,可通过FNS、IFS、F3′H等酶的催化,生成其他黄酮类化合物,如图1所示。
表1 黄酮类化合物分类
Table 1 Classification of flavonoids

类别主体结构代表物质黄酮类异黄酮类黄酮醇类二氢黄酮类查尔酮花青素二氢黄酮醇类
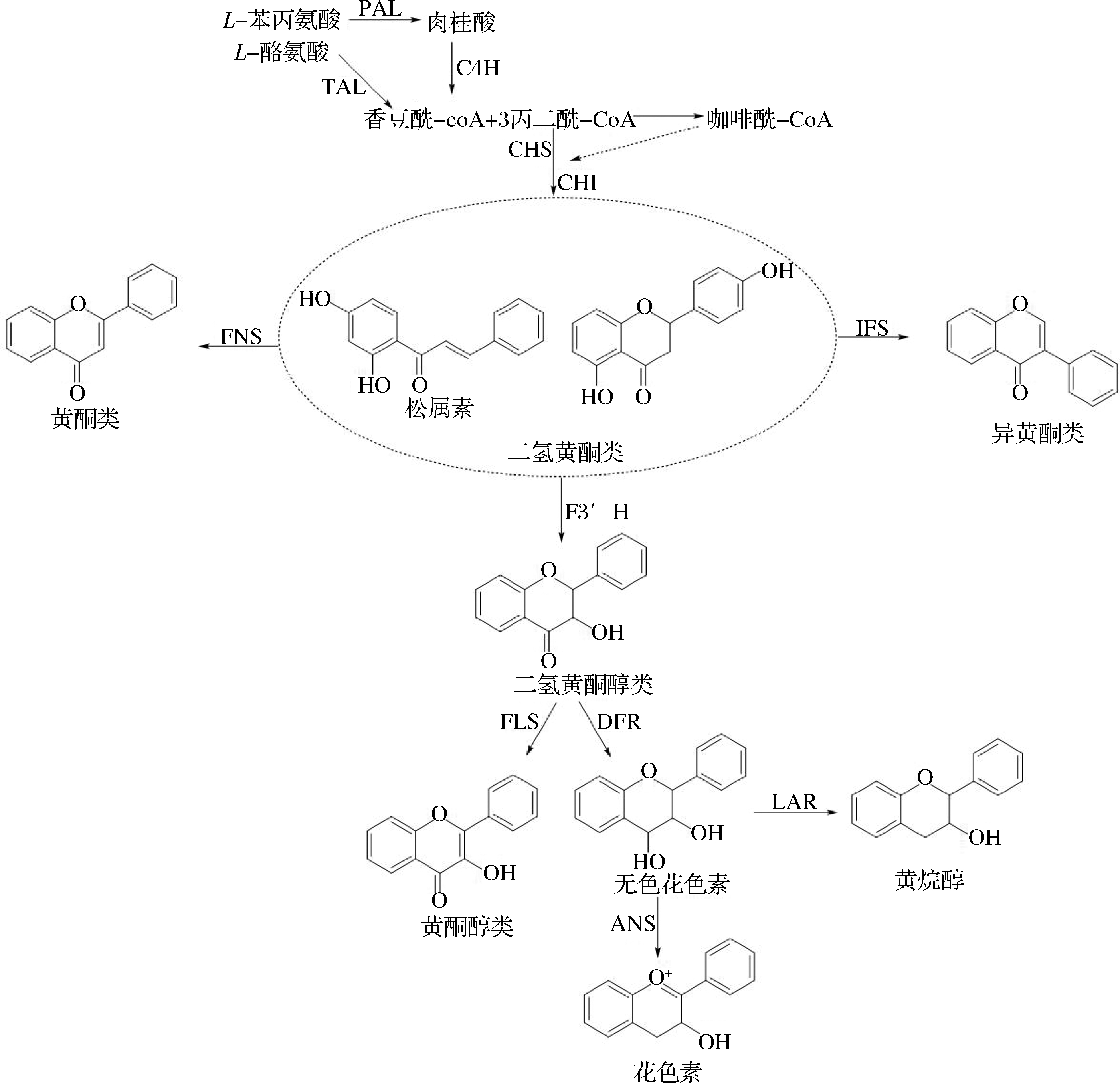
图1 黄酮类化合物生物合成途径
Fig.1 Biosynthetic pathway pf flavonoids
2 黄酮类化合物微生物基因工程合成与化学合成
2.1 黄酮类化合物微生物基因工程合成
目前,黄酮类化合物基因工程生产菌株有酿酒酵母、大肠杆菌和委内瑞拉链霉菌等[10-11]。微生物基因工程生产黄酮类化合物有生产周期短、环境污染小、可大规模生产等诸多优势。当前,很多黄酮类化合物已经在微生物中成功生产,但很多黄酮类化合物的生产都面临生产效率较低的难题。
为解决这些问题,可通过模块化共培养、调节碳源流向及基因的表达等提高黄酮类化合物产量。近年来,对黄酮类化合物模块化共培养的研究较多,最近,WANG等[12]采用模块化共培养的方法,将樱花素的合成途径分成2个模块并分别在大肠杆菌中构建相关基因,以葡萄糖为底物在间歇式反应器中扩大培养,樱花素产量达79.0 mg/L,这是目前在大肠杆菌中从头合成樱花素的最高浓度。RODRIGUEZ等[13]通过基因筛选、消除苯乙醇生物合成分支、优化核心类黄酮合成途径、补充前体2-(phosphonooxy)prop-2-en-1-olate(PEP)和 (S)-2-hydroxy-4-oxobutyl dihydrogen phosphite(E4P)、类黄酮3'羟化酶(F3H)和黄酮醇合酶(FLS)等策略在酿酒酵母中获得了86 mg/L的山奈酚,是当前在酿酒酵母中生产山奈酚的最高浓度。表2列出近年利用微生物生产黄酮类化合物相关研究。
表2 近年黄酮类化合物微生物基因工程合成及产量
Table 2 Genetic engineering synthesis and yield of flavonoids from microorganisms

此外,部分植物内生菌可通过自身代谢合成黄酮类化合物。当前,研究者已成功分离了多种有产黄酮能力的植物内生菌,该类植物内生菌多分布于镰胞霉属、曲霉属、青霉属、交链孢属、痕格孢属等。表3列出近年分离的产黄酮类化合物内生菌及其宿主植物。
表3 近年筛选产黄酮类化合物植物内生菌
Table 3 Screening of endophytic bacteria producing flavonoids in recent years

内生菌宿主植物参考文献交链孢属镰孢霉属痕格孢属灯盏花[2][25]拟茎点霉属柏木[26]曲霉属甘蔗叶[27][28]拟人盘多毛孢属红茄茗[29]Phomopsis longicollaDicerandra[30]毛霉属银杏[31]未鉴定Conyza blinii H.Lev[32]青霉属番木瓜[33]无孢菌群 越橘[34]球毛壳菌Opuntia[35]绳光黑壳戴氏霉瑞香狼毒[36]
2.2 黄酮类化合物化学合成
黄酮类化合物的化学合成受到广泛关注,主要方法有β-丙二酮酸化关环(Baker-Venkataraman,BK-VK)法、Fries重排、Auwers法和查尔酮氧化关环 (AFO)法、光催化合成等。
BK-VK法是传统的合成方法,该法利用碱催化芳甲酰卤与2-羟基苯乙酮生成酯,然后在碱处理下生成β-丙二酮,β-丙二酮再经酸催化生成黄酮类化合物。BK-VK法在实际运用中遇到过很多问题,如产物回收率低且难纯化等。为克服这些问题,在BK-VK法基础上出现了很多改良的方法,大大扩大了BK-VK的应用[37-38]。图2所示BK-VK过程。
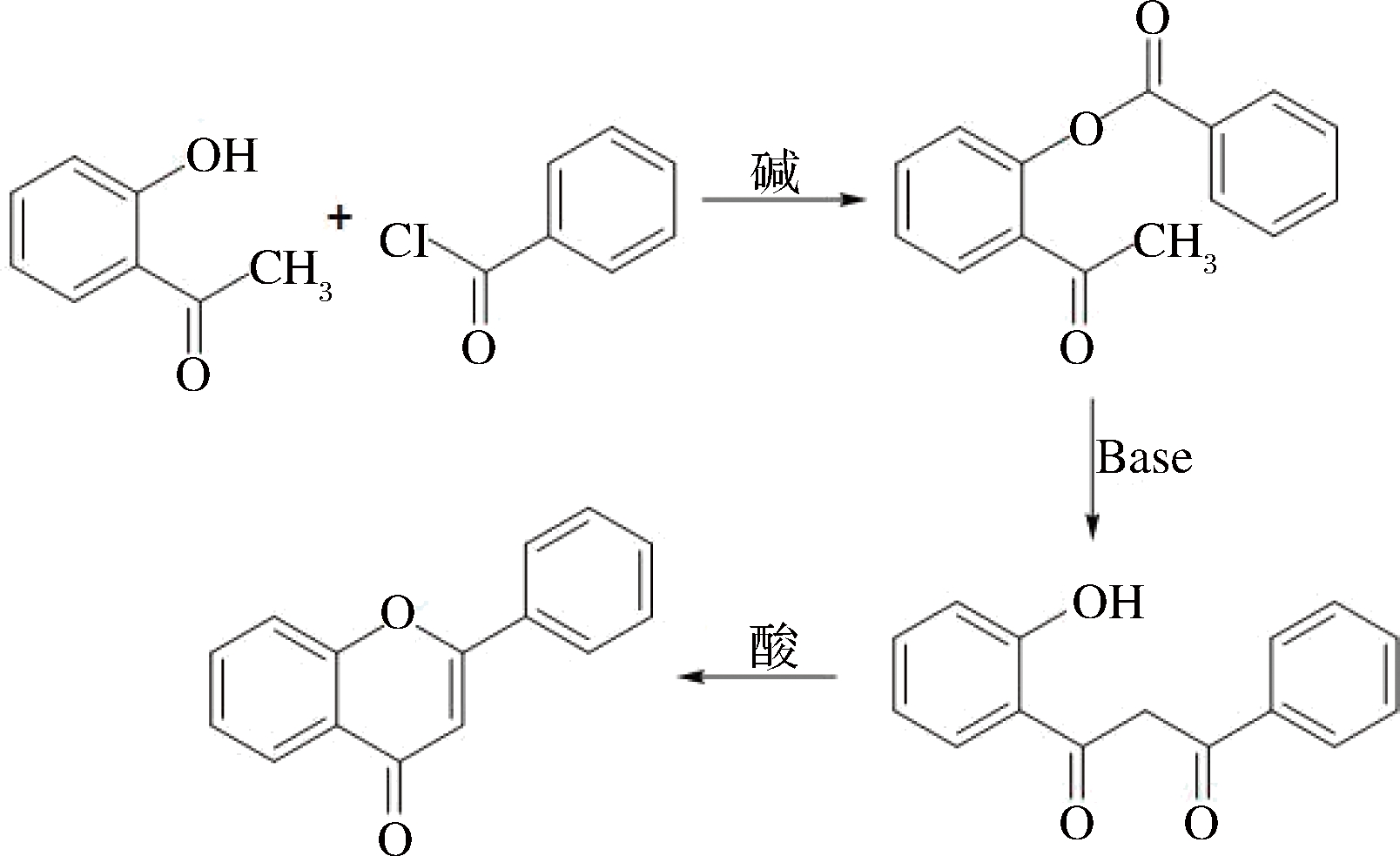
图2 β-丙二酮酸化关环法
Fig.2 Baker-Venkataraman,BK-VK
Fries重排是指酚脂在Lewis酸的催化下,酰基发生重排的过程。Fries重排常用的催化剂有三氯化铝、氯化锌、三氟化硼、氯化铁、四氯化锡、四氯化钛等,其产物有邻位和对位两种。不同催化剂,不同反应条件都会影响其产物类型,例如,四氯化钛作催化剂主要产物为邻位产物,低温更容易形成对位产物等。
黄酮类化合物合成中常用芳基丙炔酸和苯酚为底物生成酯,在吡啶溶液中滴加N,N′-二环已基碳二亚胺(DDC),所得酯再经Fries重排生成邻羟基苯乙酮, 然后经环合得到黄酮类化合物,过程如图3所示。

图3 Fries重排合成黄酮类化合物
Fig.3 Fries rearrangement for the synthesis of flavonoids
Auwers法和查尔酮氧化关环法(AFO)是比较传统的合成黄酮醇类化合物的方法。Auwers法用苯甲醛和苯并呋喃缩合再经溴化生成2溴-2-(α-溴苄基)苯并呋喃酮, 2溴-2-(α-溴苄基)苯并呋喃酮通过醇碱处理生成黄酮醇。由于Auwers法反应烦琐,产率低等原因,其实际应用较少,过程如图4所示。
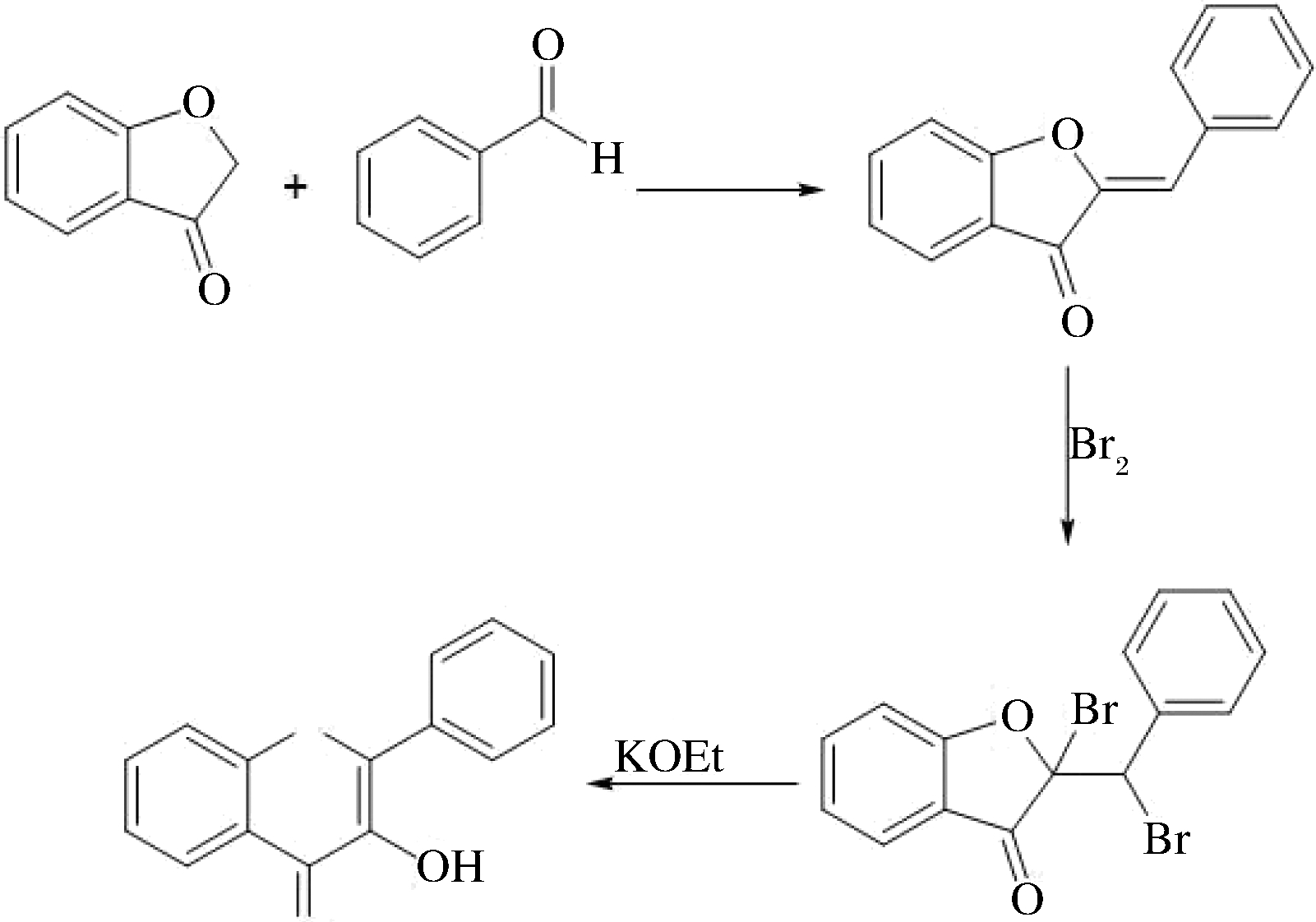
图4 Auwers法合成黄酮类化合物
Fig.4 Flavonoids were synthesized by Auwers
AFO法基本原理是2’-羟基查尔酮与H2O在碱性溶液中发生氧化环合,生成黄酮醇。其原料2’-羟基查尔酮常由苯甲醛衍生物与苯乙酮缩合生成。AFO法是经典的黄酮醇合成方法,应用较为广泛。
近年,有很多利用光辅助合成有机化合物的报道。有学者用可见光诱导叔烷基酰胺和NH4SCN发生硫氰化生成了硫代氰胺[39]。2020年,MKRTCHYAN等[40]将NH4SCN替换为BF4N2Ar和TfOIAr2,分别通过蓝光和绿光照射合成了黄酮类化合物。具体过程如图5所示。光辅助合成有绿色环保,合成效率高等优点,对光辅助合成黄酮类化合物的研究是黄酮类化合物开发利用的重要方法和手段。

图5 光辅助合成黄酮类化合物
Fig.5 Photoassisted synthesis of flavonoids
3 黄酮类化合物结构修饰
目前对黄酮类化合物结构修饰的主要方法有生物法和化学法。生物法主要是利用微生物分泌的酶进行修饰,化学修饰则是通过特定化学反应进行。修饰位点主要集中在C环2、3位,A环5、6、7、8位和B环2′、3′、4′ 位,主要有糖基化、去糖基化、酰基化、羟基化、甲基化及金属离子等。
3.1 微生物修饰
目前,已发现了许多对黄酮类化合物有修饰功能的微生物,其中羟基化、去羟基化、甲基化大多发生在B环的C3和C4位点,糖基化和去糖基化多发生在C环。表4为相关微生物对黄酮类化合物的修饰作用。
表4 黄酮类化合物微生物修饰
Table 4 Microbial modification of flavonoids

修饰类别微生物或催化酶参考文献糖基化尖芽孢杆菌、链球菌、I.fumosorosea KCH J2[41][42][43]去糖基化酿酒酵母、乳杆菌、双歧杆菌[44][45][46]酰基化南极假丝酵母脂肪酶B[47]甲基化去甲基化白僵菌、黑曲霉[48][49]羟基化羰基还原黑曲霉[50]
3.2 化学修饰
黄酮类化合物化学修饰的主要方法有金属离子螯合、卤族元素和活性基团的引入等。通过化学结构的修饰,可直接影响黄酮类化合物的理化性质和生物活性。
黄酮类化合物对金属离子有较强的螯合作用,其配合物的配体可与金属离子产生协同作用,增加其生物活性。很多黄酮类化合物如芦丁、大黄素、槲皮素、黄芩苷等均有金属离子螯和及其生物活性研究的报道,其中铜、锌以及稀土金属元素与黄酮类化合物的螯和报道较多。图6所示部分常见黄酮类化合物金属离子螯和位点。
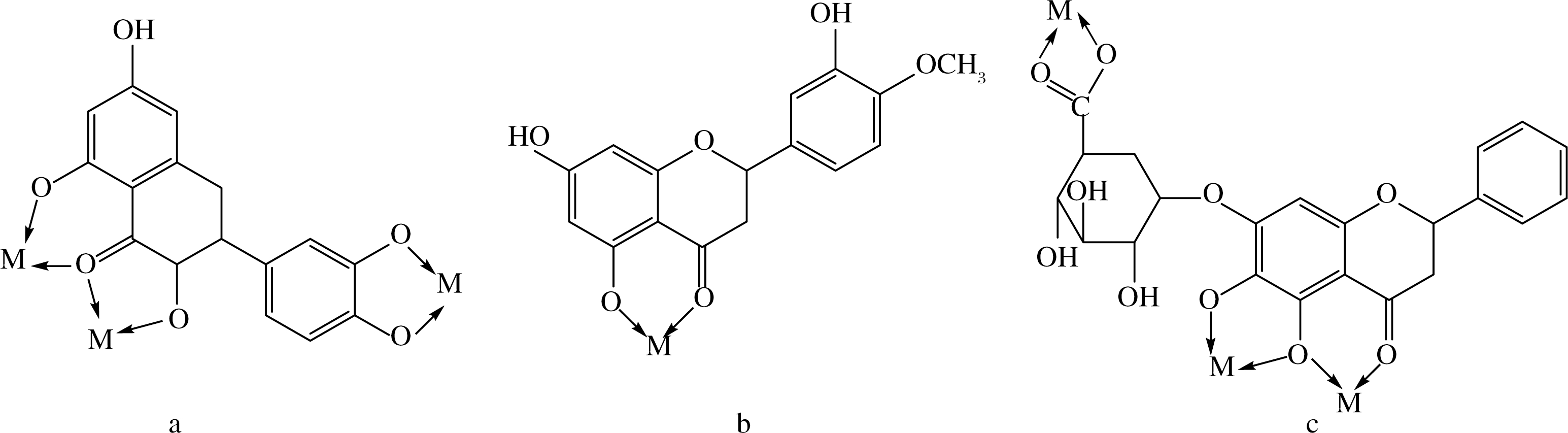
a-槲皮素;b-香叶木素;c-黄芩苷
图6 常见黄酮类化合物金属螯和位点
Fig.6 Metal chelates sites of common flavonoid compounds
卤族元素引入黄酮分子可提高黄酮类化合物生物活性,引入元素主要是氟、氯、溴三种。其引入后物质的活性大小通常是氟>氯>溴[51]。
活性基团的引入主要是甲基、酰基和羟基等。在黄酮分子中引入不同活性基团,可改变黄酮分子构象,增强其生物活性,甚至产生新的生理功能。COPMANS等[52]研究了 naringenin、山奈酚及其3种甲基化衍生物对斑马鱼癫痫发作(ptz诱导)的作用,结果表明,甲基化黄烷酮NRG-dm对斑马鱼幼体ptz诱导的癫痫发作效果非常好。羟基的引入可增大黄酮类化合物水溶性和抗氧化活性,其引入位点不同,对黄酮类化合物活性的影响也不同,其相对活性大小和引入位点的关系为:7>4′≈3>3′>> 5[53]
4 展望
黄酮类化合物在植物中含量低,不易获得。对生物和化学方法合成黄酮类化合物理论和技术的拓展,可望成为解决这一问题的有效途径。基因工程及化学方法合成黄酮类化合物可为黄酮类化合物更广泛和深入的应用提供可能,发掘和完善黄酮类化合物合成及修饰方法,有望弥补植物中黄酮类化合物含量低,生物利用度低等问题。此外,黄酮类化合物对医药和食品等领域的意义重大,将基因工程和化学方法广泛用于新型黄酮类化合物的开发利用,其意义不可估量。
[1] 徐任生.天然产物化学[M].第二版.北京:科学出版社,2004.
XU R S.Chemistry of natural product[M].2nd ed.Beijing:Science Press,2004.
[2] 杨梦莉,赖泳红,郭凤根,等.灯盏花内生真菌分离及其产黄酮内生真菌的初步研究[J].西南农业学报,2018,31(2):318-321.
YANG M L,LAI Y H,GUO F G,et al.Isolation and selection of flavones-forming endophytic fungi from Erigeron breviscapus[J].Southwest China Journal of Agricultural Sciences,2018,31(2):318-321.
[3] XIAO Y,HAN F,LEE I S.Microbial transformation of licochalcones[J].Molecules,2020,25(1):60.
[4] MUKHERJEE A,MISHRA S,KOTLA N K,et al.Semisynthetic quercetin derivatives with potent antitumor activity in colon carcinoma[J].Acs Omega,2019,4(4):7 285-7 298.
[5] LI Y,LI Y,HE J,et al.The relationship between pharmacological properties and structure-activity of chrysin derivatives[J].Mini Reviews in Medicinal Chemistry,2019,19(7):555-568.
[6] 金永生,刘超美,吴秋业,等.新型金雀异黄素衍生物 5-羟基-4′-硝基-7-取代酰氧基异黄酮的合成及抗肿瘤活性研究[J].第二军医大学学报,2005,26(2):182-185.
JIN Y S,LIU C M,WU Q Y,et al.Design and synthesis of genistein derivatives 5-hydroxy-4′-nitro-7-substituted-acyloxo-isoflavone and their antitumor effects[J].Academic Journal of Second Military Medical University,2005,26(2):182-185.
[7] 刘建中,翁玲玲,郑虎.异黄酮哌嗪衍生物的合成[J].中国药物化学杂志,2000(1):49-51;56.
LIU J Z,WENG L L,ZHENG H.Synthesis of isoflavone piperazine derivatives[J].Chinese Journal of Medicinal Chemistry,2000(1):49-51;56.
[8] 邹丽秋,王彩霞,匡雪君,等.合成途径及合成生物学研究进展[J].中国中药杂志.2016.41(22):4 124-4 128.
ZOU L Q,WANG C X,KUANG X J,et al.Advance in flavonoids biosynthetic pathway and synthetic biology[J].China Journal of Chinese Materia Medica,2016,41(22):4 124-4 128.
[9] NABAVI S M, AMEC D,TOMCZYK M,et al.Flavonoid biosynthetic pathways in plants:Versatile targets for metabolic engineering[J].Biotechnology Advances,2020,38:107 316.
AMEC D,TOMCZYK M,et al.Flavonoid biosynthetic pathways in plants:Versatile targets for metabolic engineering[J].Biotechnology Advances,2020,38:107 316.
[10] PANDEY R P,PARAJULI P,KOFFAS M A G,et al.Microbial production of natural and non-natural flavonoids:Pathway engineering,directed evolution and systems/synthetic biology[J].Biotechnology Advances,2016,34(5):634-662.
[11] TRANTAS E A,KOFFAS M A G,XU P,et al.When plants produce not enough or at all:Metabolic engineering of flavonoids in microbial hosts[J].Frontiers in Plant Science,2015,6:7.
[12] WANG X,LI Z,POLICARPIO L,et al.De novo biosynthesis of complex natural product sakuranetin using modular co-culture engineering[J].Applied Microbiology and Biotechnology,2020:104(11):1-13.
[13] RODRIGUEZ A,STRUCKO T,STAHLHUT S G,et al.Metabolic engineering of yeast for fermentative production of flavonoids[J].Bioresource Technology,2017,245:1 645-1 654.
[14] HWANG E I,KANEKO M,OHNISHI Y,et al.Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster[J].Applied and Environmental Microbiology,2003,69(5):2 699-2 706.
[15] KOOPMAN F,BEEKWILDER J,CRIMI B,et al.De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae[J].Microbial Cell Factories,2012,11(1):155.
[16] TRANTAS E,PANOPOULOS N,VERVERIDIS F.Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae[J].Metabolic Engineering,2009,11(6):355-366.
[17] LV Y,EDWARDS H,ZHOU J,et al.Combining 26s rDNA and the Cre-loxP system for iterative gene integration and efficient marker curation in Yarrowia lipolytica[J].ACS Synthetic Biology,2019,8(3):568-576.
[18] PARK S R,YOON J A,PAIK J H,et al.Engineering of plant-specific phenylpropanoids biosynthesis in Streptomyces venezuelae[J].Journal of Biotechnology,2009,141(3-4):181-188.
[19] MIYAHISA I,KANEKO M,FUNA N,et al.Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster[J].Applied Microbiology and Biotechnology,2005,68(4):498-504.
[20] MIYAHISA I,FUNA N,OHNISHI Y,et al.Combinatorial biosynthesis of flavones and flavonols in Escherichia coli[J].Applied Microbiology and Biotechnology,2006,71(1):53-58.
[21] ZHU S,WU J,DU G,et al.Efficient synthesis of eriodictyol from L-tyrosine in Escherichia coli[J].Applied and Environmental Microbiology,2014,80(10):3 072-3 080.
[22] LIM C G,FOWLER Z L,HUELLER T,et al.High-yield resveratrol production in engineered Escherichia coli[J].Applied and Environmental Microbiology,2011,77(10):3 451-3 460.
[23] KIM M J,KIM B G,AHN J H.Biosynthesis of bioactive O-methylated flavonoids in Escherichia coli[J].Applied Microbiology and Biotechnology,2013,97(16):7 195-7 204.
[24] WATTS K T,LEE P C,SCHMIDT-DANNERT C.Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli[J].BMC Biotechnology,2006,6(1):22.
[25] LU Y,YE C,CHE J,et al.Genomic sequencing,genome-scale metabolic network reconstruction,and in silico flux analysis of the grape endophytic fungus Alternaria sp.MG1[J].Microbial Cell Factories,2019,18(1):13.
[26] ISAKA M,JATURAPAT A,RUKSEREE K,et al.Phomoxanthones A and B,novel xanthone dimers from the endophytic fungus Phomopsis species[J].Journal of Natural Products,2001,64(8):1 015-1 018.
[27] TIAN X,HUANG C,HUANG J.Identification of flavonoids and flavonoid-producing endophytic fungi isolated from Opisthopappus taihangensis (Ling) C.Shih[J].Bangladesh Journal of Botany,2017,46(3):1 063-1 070.
[28] 周宁.甘蔗叶产黄酮内生真菌的筛选与鉴定[D].南宁:广西大学,2017.
ZHOU N.Screening and identification of flavonoids-producing endophytic fungi from sugarcane leaves[D].Nanning:Guangxi University,2017.
[29] XU J,KJER J,SENDKER J,et al.Chromones from the endophytic fungus Pestalotiopsis sp.isolated from the Chinese mangrove plant Rhizophora mucronata[J].Journal of Natural Products,2009,72(4):662-665.
[30] WAGENAAR M M,CLARDY J.Dicerandrols,new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint[J].Journal of Natural Products,2001,64(8):1 006-1 009.
[31] 张海龙,李善春,卢维浩,等.银杏内生真菌多样性与产黄酮类物质真菌的分离和鉴定[J].土壤,2015,47(1):135-141.
ZHANG H L,LI S C,LU W H,et al.Distribution and identification of flavonoid-producing endophytic Fungi in Ginkgo biloba L[J].Soils,2015,47(1):135-141.
[32] TANG Z,WANG Y,YANG J,et al.Isolation and identification of flavonoid-producing endophytic fungi from medicinal plant Conyza blinii H.Lév that exhibit higher antioxidant and antibacterial activities[J].PeerJ,2020,8:e8978.
[33] 柯树炜,吕金慧,陈萍,等.产黄酮番木瓜内生真菌的筛选和鉴定[J].生物技术通报,2019,35(3):47-52.
KE S W,LV J H,CHEN P,et al.Isolation and identification of flavonoids-producing endophytic fungi in papaya[J].Biotechnology Bulletin,2019,35(3):47-52.
[34] 李姝诺,李亚东,王琦,等.越橘产黄酮类化合物内生真菌的筛选[J].吉林农业大学学报,2009,31(5):587-591.
LI S N,LI Y D,WANG Q,et al.Bolting flavonoid-producing endophytic fungi from Vaccinium[J].Journal of Jilin Agricultural University,2009,31(5):587-591.
[35] WIJERATNE E M K,TURBYVILLE T J,FRITZ A,et al.A new dihydroxanthenone from a plant-associated strain of the fungus Chaetomium globosum demonstrates anticancer activity[J].Bioorganic & Medicinal Chemistry,2006,14(23):7 917-7 923.
[36] 王欢.瑞香狼毒内生真菌分离鉴定与发酵产物黄酮的检测[D].杨凌:西北农林科技大学,2016.
WANG H.Isolation and identification of fungal endophytes of Stellera chamaejasme L.and testing for flavonoids produced through fermentation[D].Yangling:Northwest A&F University,2016.
[37] 康帅涛,李晓东,许环军,等.黄酮及其衍生物的高效合成和抗肿瘤活性[J].沈阳药科大学学报,2011,28(11):862-867;881.
KANG S L,LI X D,XU H J,et al.Efficient synthesis and antitumor activities of flavone and its derivatives[J].Journal of Shenyang Pharmaceutical University,2011,28(11):862-867;881.
[38] 段新方,张站斌,段新红.5,3′,4′-三羟基-6,7-二甲氧基黄酮的另法全合成[J].有机化学,2003(4):353-355.
DUAN X F,ZHANG Z B,DUAN X H.Modified Synthesis of 5,3′,4′-trihydroxyl-6,7-dimethoxyflavone[J].Chinese Journal of Organic Chemistry,2003(4):353-355.
[39] CHAUDHARY A K,HWANG I Y,JO Y J,et al.Enzymatic synthesis of amentoflavone glycoside using recombinant oleandomycin glycosyltransferase[J].Journal of Industrial and Engineering Chemistry,2015,25:304-307.
[40] MKRTCHYAN S,IAROSHENKO V O.Visible-light-mediated arylation of ortho-hydroxyarylenaminones:Direct access to isoflavones[J].Chemical Communications,2020,56(17):2606-2609.
[41] GAO C,MAYON P,MACMANUS D A,et al.Novel enzymatic approach to the synthesis of flavonoid glycosides and their esters[J].Biotechnology and Bioengineering,2000,71(3):235-243.
[42] CHAUDHARY A K,HWANG I Y,JO Y J,et al.Enzymatic synthesis of amentoflavone glycoside using recombinant oleandomycin glycosyltransferase[J].Journal of Industrial and Engineering Chemistry,2015,25:304-307.
[43] DYMARSKA M,JANECZKO T,KOSTRZEWA-SUS OW E.Glycosylation of methoxylated flavonoids in the cultures of Isaria fumosorosea KCH J2[J].Molecules,2018,23(10):2 578.
OW E.Glycosylation of methoxylated flavonoids in the cultures of Isaria fumosorosea KCH J2[J].Molecules,2018,23(10):2 578.
[44] MANDALARI G,BENNETT R N,BISIGNANO G,et al.Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel,a byproduct of the essential oil industry[J].Journal of Applied Microbiology,2007,103(6):2 056-2 064.
[45] WEI Q K,CHEN T R,CHEN J T.Using of Lactobacillus and Bifidobacterium to product the isoflavone aglycones in fermented soymilk[J].International Journal of Food Microbiology,2007,117(1):120-124.
[46] GAYA P,PEIROTÉN  ,LANDETE J M.Transformation of plant isoflavones into bioactive isoflavones by lactic acid bacteria and Bifidobacteria[J].Journal of Functional Foods,2017,39:198-205.
,LANDETE J M.Transformation of plant isoflavones into bioactive isoflavones by lactic acid bacteria and Bifidobacteria[J].Journal of Functional Foods,2017,39:198-205.
[47] GAYOT S,SANTARELLI X,COULON D.Modification of flavonoid using lipase in non-conventional media:Effect of the water content[J].Journal of Biotechnology,2003,101(1):29-36.
[48] ARA JO K C F,DE MB COSTA E M,PAZINI F,et al.Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products[J].Food and Chemical Toxicology,2013,51:93-96.
JO K C F,DE MB COSTA E M,PAZINI F,et al.Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products[J].Food and Chemical Toxicology,2013,51:93-96.
[49] BUISSON D,QUINTIN J,LEWIN G.Biotransformation of polymethoxylated flavonoids:access to their 4′-O-demethylated metabolites[J].Journal of Natural Products,2007,70(6):1 035-1 038.
[50] KOSTRZEWA-SUS OW E,JANECZKO T.Microbial transformations of 5-hydroxy-and 5-methoxyflavone in Aspergillus niger and Penicillium chermesinum cultures[J].Journal of Microbiology,Biotechnology and Food Sciences,2018,3(6):448-452.
OW E,JANECZKO T.Microbial transformations of 5-hydroxy-and 5-methoxyflavone in Aspergillus niger and Penicillium chermesinum cultures[J].Journal of Microbiology,Biotechnology and Food Sciences,2018,3(6):448-452.
[51] DIAS T A,DUARTE C L,LIMA C F,et al.Superior anticancer activity of halogenated chalcones and flavonols over the natural flavonol quercetin[J].European Journal of Medicinal Chemistry,2013,65:500-510.
[52] COPMANS D,ORELLANA-PAUCAR A M,STEURS G,et al.Methylated flavonoids as anti-seizure agents:Naringenin 4′,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models[J].Neurochemistry International,2018,112:124-133.
[53] 梅青刚,袁伟成,王淳.黄酮醇类化合物的合成研究进展[J].有机化学,2015,35(1):70-84.
MEI Q G,YUAN W C,WANG C.Progress in the synthesis of 3-hydroxyflavones[J].Chinese Journal of Organic Chemistry,2015,35(1):70-84.