黄浆水是白酒固态发酵过程中,沉积在窖池底部的棕黄色黏稠液体,含有酒精、水分及各种香味物质等[1-2]。将黄浆水酯化用于白酒勾兑,不仅能弥补白酒风味成分的不足,还能避免人工添加香精产生的“浮香”问题[3-4]。但黄浆水酯化液中水的含量在90%以上,如何提取酯类物质是应用的关键。目前常见的提取方法有蒸馏[5]和超临界CO2流体萃取[6],该现有方法均存在设备投入大,萃取成本高[7-8],无法满足实际生产应用的问题。
双水相体系如乙醇/异丙醇/丙酮等亲水有机溶剂和水的混溶体系在(NH4)2SO4、Na2CO3、K2HPO4、NaH2PO4、K3PO4等无机盐的作用下出现分层以达到分离作用。PRATIWI等[9]利用乙醇/硫酸铵体系萃取琥珀酸;XU等[10]利用1,4-二恶烷/Na2SO4体系萃取乳酸;姚誉阳等[11]采用PEG-NaH2PO4体系萃取头孢呋辛酯。双水相萃取具有较高的生物相溶性、易于放大、可连续化操作,已经应用于蛋白质、核酸、氨基酸、抗生素以及色素等产品的分离和纯化,必将在生物工程、食品等领域广泛应用[12-14]。
本文采用双水相萃取分离提取洋河酒厂黄浆水酯化液中的酯类,利用酯化液中本身含有的乙醇进行成相,研究确定最合适的萃取用盐,优化双水相的萃取工艺。范文来等[15]研究表明,绵柔型白酒的关键风味酯类有己酸乙酯、戊酸乙酯、丁酸乙酯、辛酸乙酯和乙酸乙酯,并考察了该5种风味酯类在双水相体系中的分配系数和萃取率。对萃取液进一步处理得到调味液粗产品,与黄浆水的理化性质和天之蓝白酒的风味成分对比,考察双水相萃取酯类的效果。基于该研究为黄浆水酯化液的高效利用提供理论指导。
1 材料与方法
1.1 材料与试剂
黄浆水,江苏洋河酒厂;黄浆水酯化液,酯化制备;NaH2PO4、Na2CO3、(NH4)2SO4、K2HPO4、无水乙醇(均为分析纯),己酸乙酯、辛酸乙酯、丁酸乙酯、乙酸乙酯、戊酸乙酯(均为色谱纯),国药沃凯化学试剂有限公司。
1.2 仪器与设备
GCMS-QP2020气质联用仪(搭配HS-10自动顶空进样器)、SH-Rtx-Wax毛细管色谱柱(30 m×0.25 mm×0.25 μm),日本SHIMADZU公司。
1.3 风味酯类分析方法
样品前处理[3]:用去离子水将样品稀释10倍,取8 mL加入20 mL顶空瓶中,加入3 g NaCl,振荡使水溶液过饱和,压紧瓶盖。
顶空-GC-MS定性分析[16-17]:以SH-Rtx-Wax毛细管色谱柱对样品进行分离。GC升温程序:50 ℃保持2 min,以5 ℃/min的速度升温至90 ℃,保持0 min,再以10 ℃/min的速度升温至230 ℃,保持7 min;进样口温度250 ℃,载气He,流速2 mL/min,进样量1 μL,不分流进样;MS条件:EI,电离能量70 eV,灯丝电流设定值 120 μA;离子源温度200 ℃,扫描方式:SIM。
顶空-气相法定量分析:DB-Wax毛细管色谱柱对样品进行分离,检测器为FID氢火焰离子检测器,外标法定量。自动顶空进样器程序:恒温炉60 ℃,样品流路温度100 ℃,传输线温度100 ℃,GC循环35 min。
1.4 无机盐/乙醇双水相平衡相图的绘制
采用浊点滴定法[18]:取50 mL具塞刻度试管若干,加入10 mL去离子水;准确称取不同质量的无机盐分别加入试管中,涡旋使之充分溶解,形成不同质量分数的无机盐-水溶液;将无水乙醇注入酸式滴定管,对无机盐-水溶液进行滴定,以气泡消失溶液恰好变为乳浊液为滴定终点,记下无机盐的质量m以及对应的乙醇消耗量V。以无机盐质量分数为横坐标轴,浊点乙醇体积分数为纵坐标轴,绘制平衡相图。
1.5 双水相体系中相比、分配系数和萃取率的测定
结合参考文献[18-19],改进试验方法和计算公式,准确称取不同质量的无机盐(精确到0.000 1 g),置于20 mL具塞刻度试管中;加入黄浆水酯化液15 g,使每只试管的总体积约为15 mL,形成不同盐浓度梯度(根据相图,盐浓度高于最低加盐量)的萃取体系;涡旋振荡5~10 min使无机盐充分溶解并与酯化液充分混合,静置过夜;待分层充分稳定后,分别读取上下相的体积,计算相比R;分别吸取上下相0.8 mL,稀释10倍,对5种主要风味酯类(己酸乙酯、辛酸乙酯、丁酸乙酯、戊酸乙酯、乙酸乙酯)的浓度进行定量分析,相比R、各酯类分配系数K、萃取率Y计算如公式(1)~公式(3)所示:
(1)
(2)
(3)
式中:Vt、Vb,分别为上下相的体积,mL;Ct、Cb,分别为酯化物在上相和下相中的质量浓度,mg/L。
1.6 萃取液进一步脱水除杂
用分液漏斗分离萃取后得到上相液体,缓慢添加无水Na2SO4粉末直至不再产生絮状沉淀,再加少量无水Na2SO4,振荡后静置过夜;离心弃去沉淀,硅藻土过滤后即为调味液粗产品(简称调味液),测定调味液的理化指标和风味成分。
2 结果与分析
2.1 双水相体系的选取
根据预实验选取成相较好的4种无机盐K2HPO4、NaH2PO4、Na2CO3、(NH4)2SO4,分别绘制与乙醇的相平衡关系相图,如图1所示。实线以上是两相区,以下是均相区;图中虚线是相图的连接线,上相T主要含乙醇,下相B主要含无机盐。点M表示体系分相后,上相体积与线段TM的乘积等于下相体积与线段MB的乘积。当乙醇含量一定时,无机盐的含量越高,相比R(即MB/TM)越小,上相所含乙醇越多无机盐越少,下相则相反。当连接线越偏离曲线时,上下相组分差异越大,则有更好的分离效果;当平衡曲线的曲率越大,偏离连结线的程度越深,分相越快越明显。K2HPO4的平衡曲线弯曲程度最深,Na2CO3次之,(NH4)2SO4的平衡曲线最接近连接线。另外,K2HPO4的溶解度较大(20 ℃,62 g/100g水),可控范围较广,20 ℃左右溶解性平稳,双水相体系受温度的影响较小。Na2CO3在20~30 ℃溶解度相差20 g左右。因此,选用分相能力最强、性质较稳定的K2HPO4作为双水相萃取的分相用盐,这与范蕊庆等[20]和ZHANG等[12]选取的萃取盐相同。
2.2 双水相萃取酯化液中风味酯类的工艺优化
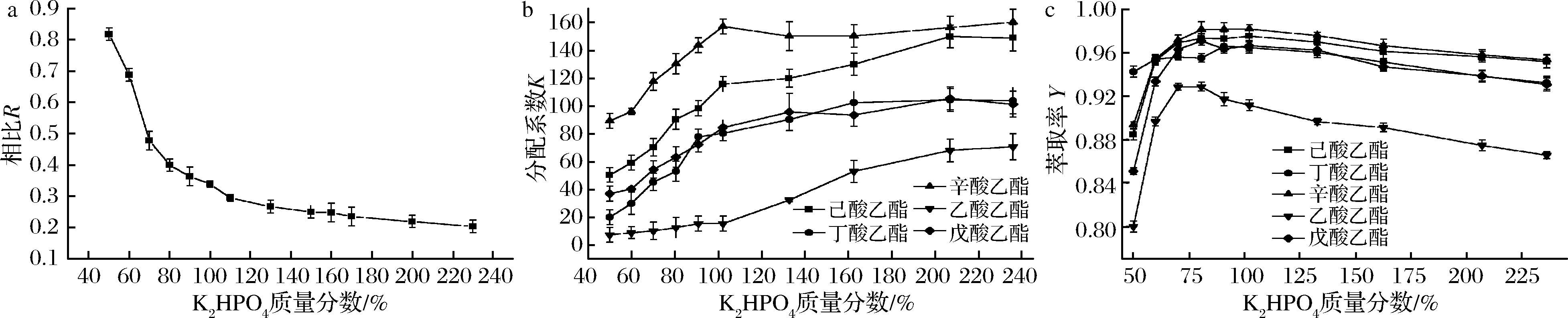
2.2.1 K2HPO4浓度对双水相萃取酯类的影响
酯化液的乙醇体积分数为20%左右,加入不同质量分数的K2HPO4,考察K2HPO4浓度对相比R、分配系数K和萃取率Y的影响。由图2可知,随着K2HPO4质量分数的增大,相比R急速下降后趋于平缓,5种酯类物质的分配系数K随之增大,萃取率Y呈先上升后下降的趋势,在无机盐质量分数为80%左右时达到最大值,5种酯类的萃取率均达到90%~99%。当K2HPO4的浓度较低时,与乙醇争夺水分子的能力较弱,使得大量的水分聚集在上层,上下相组分差异较小,极性相差不大,对酯类选择性不强,分配系数相对较低;随着K2HPO4浓度升高,盐析效应和结合水能力增强,K2HPO4夺走了酯类周围的水分子而将酯类排挤出水相(盐相)而进入上相(乙醇相),相比R下降,上下相组分差异增大,对酯类的选择性增强,分配系数K也显著增大[8]。
不同酯类K值的大小关系为:辛酸乙酯>己酸乙酯>戊酸乙酯>丁酸乙酯>乙酸乙酯,这与它们的极性呈负相关,溶剂的极性代表疏水力的强弱,极性越强越易溶于水,而极性越弱则较为难溶而易于分层。一般情况下,可以用介电常数来度量溶剂相对极性的强弱,结合表1中5种酯类化合物的介电常数和溶解性[21],验证了双水相体系对酯类的分离效果取决于其疏水作用的强弱。

a-K2HPO4/乙醇;b-(NH4)2SO4/乙醇;c-Na2CO3/乙醇;d-NaH2PO4/乙醇
图1 无机盐/乙醇双水相体系相图
Fig.1 Phase diagram of inorganic salt/ethanol aqueous system
a-相比R;b-分配系数K;c-萃取率Y
图2 K2HPO4质量分数对双水相体系相比R、分配系数K和萃取率Y的影响
Fig.2 Effect of the K2HPO4 mass fraction on the phase ratio R, the partition coefficient K and the extraction rate Y in aqueous two-phase system
表1 五种酯类化合物的相对介电常数和溶解性
Table 1 Relative permittivity and solubility of five ester compounds

项目乙酸乙酯丁酸乙酯戊酸乙酯己酸乙酯辛酸乙酯测试温度/℃251920--相对介电常数(εr)65.14.7--水溶性微溶微溶微溶不溶不溶乙醇中溶解性互溶互溶易溶易溶易溶
注:-表示无意义或没有确切资料
2.2.2 pH对双水相萃取酯类的影响
酯化液含有未被酯化的有机酸,其pH值在4.5左右,调节pH值至3.5、4.5、6.0、7.5、8.5、9.5、10.5,加入质量分数为80%的K2HPO4,考察不同pH对相比R和己酸乙酯的分配系数K的影响,如图3所示。pH对相比R和己酸乙酯分配系数K无显著影响。古博华[19]研究表明硫酸铵/丙酮双水相体系萃取丁二酸中,萃取率随pH值增大而减小,可能缘于pH值的增大使越来越多的丁二酸生成盐,易溶解于水相(下相)而几乎不溶于丙酮;而本文中H+浓度并不影响酯类在体系中的存在形式,且萃取盐K2HPO4是缓冲盐,酯化液中有机酸含量较低,K2HPO4本身具有稳定pH的性质,因此,选择双水相体系的pH值为4.5。
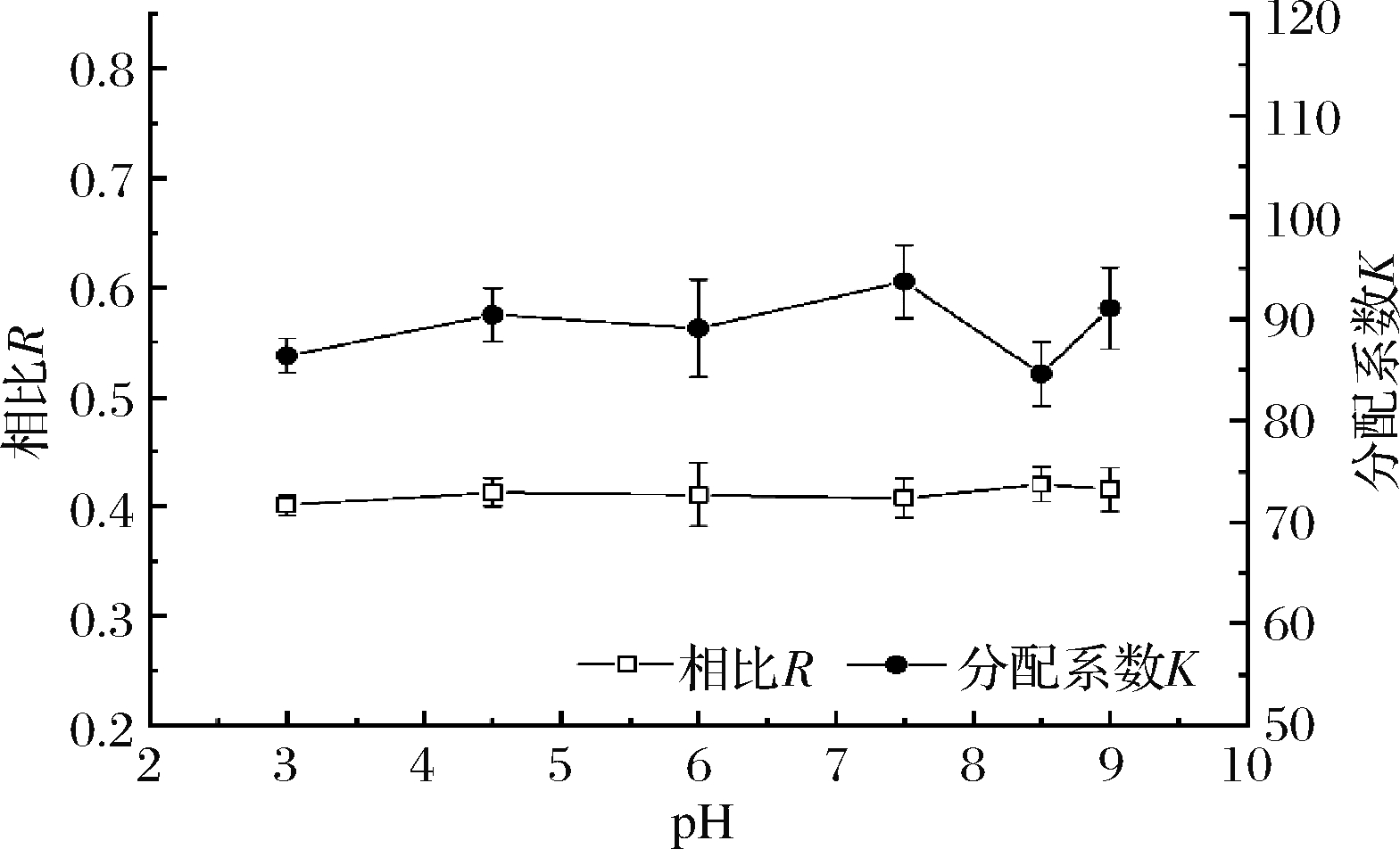
图3 不同pH对双水相体系的相比R和己酸乙酯的 分配系数K的影响
Fig.3 Effect of pH on the phase ratio R and the partition coefficient K of ethyl hexanoate in aqueous two-phase system
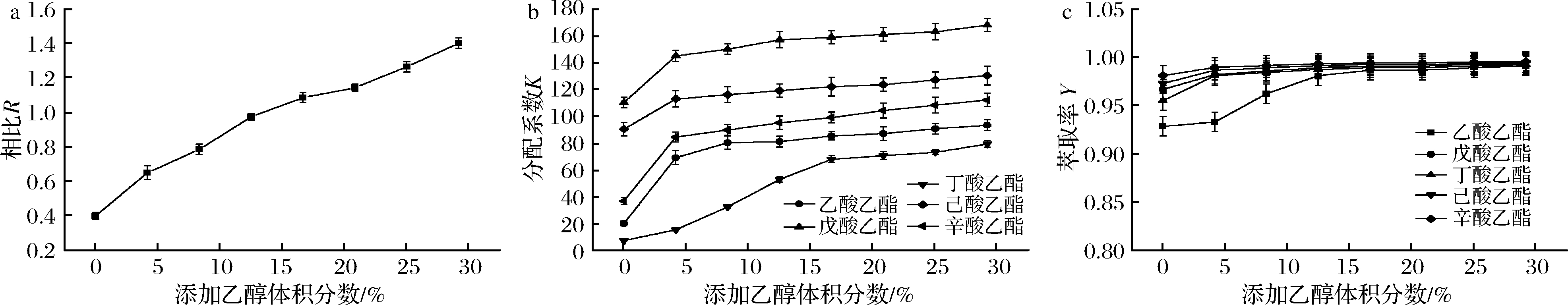
2.2.3 乙醇含量对双水相萃取酯类的影响
双水相体系中K2HPO4质量分数为80%,酯化液中原乙醇体积分数为20%,考察添加无水乙醇的含量对R、K和Y的影响。如图4所示,随乙醇含量增大,上相的体积也随之增大,相比R增大;K值随乙醇的含量增大而上升,当乙醇添加量达20%后趋势平缓;乙醇含量的增加对酯类的萃取效果有一定影响[18],除极性较大的乙酸乙酯萃取率从93%提高至99%,4种酯类萃取率均变化不大,为95%~100%。添加乙醇对上相有稀释作用,酯类的浓度随之降低,对萃取率无显著提升的作用,考虑到节省溶剂,避免后续去除溶剂对酯类造成的损失,双水相体系应不添加额外乙醇,以酯化液中乙醇体积分数20%为较优条件。
a-相比R;b-分配系数K;c-萃取率Y
图4 乙醇添加量对双水相体系相比R、分配系数K和萃取率Y的影响
Fig.4 Effect of ethanol content on the phase ratio R, the partition coefficient K and the extraction rate Y in aqueous two-phase system
综上所述,本文确定了双水相萃取酯化液中风味酯类的较优工艺为:酯化液15 g,K2HPO4质量分数为80%,乙醇体积分数为20%,pH值为4.5,在该条件下,5种酯类的萃取率均达到90%~99%,这与XU等[10]的乳酸萃取率最高达91%和姚誉阳等[11]的头孢呋辛酯萃取率92%的萃取效果接近;该萃取率高于裴莹等[22]的α-酮戊二酸萃取率80.45%和PRATIWI等[9]的琥珀酸萃取率82%,证明了双水相体系对酯化液中的酯类有较好的萃取效果。
2.3 调味液理化性质和风味化合物分析
2.3.1 黄浆水和调味液理化性质分析
黄浆水和调味液的基本理化性质如表2所示,黄浆水酯化、萃取和脱水处理后,水分含量从91.11%降低至1.14%,乙醇含量高达98.96%,萃取效果显著,用该调味液进行勾兑不会降低基酒的酒精度;调味液总酯含量(67.85 g/L)显著高于黄浆水(1.37 g/L),总酸几乎未检出。此外,在双水相萃取的过程中,残余蛋白、糖类、淀粉、醛类等含有亲水基团被留在下相而去除;单宁及色素等多酚类物质更易溶于醇相而被富集[3]。
表2 黄浆水和调味液的理化性质
Table 2 The physical and chemical properties of yellow water and the blending liquid

指标黄浆水调味液指标黄浆水调味液pH3.27.91酸度/(mg·L-1)4.47未检出蛋白质/%0.13未检出总酸(以己酸计)/(g·L-1)53未检出水分/%91.111.14固形物/%4.60.03还原糖/(g·L-1)0.80.30总糖(以葡萄糖计)/(g·L-1)8.60.31残余淀粉/(g·L-1)7.70.01总酯(以己酸乙酯计)/(g·L-1)1.3767.85CODCr/(mg·L-1)49 000未计量乙醇体积分数/%4.598.96总氮/%0.102未检出单宁及色素/%0.150.69
2.3.2 萃取液、调味液和洋河白酒风味成分的对比
由表3可知,双水相萃取有效地富集了风味酯类、醇类和一些酚类化合物,萃取液和调味液的酯类质量浓度显著高于酯化液,且萃取液中5种酯类的质量浓度是酯化液的3~5倍;另外,萃取液和调味液中有机酸均未检出;部分短链有机酸易溶于水相被除去,另外,成相用盐K2HPO4是弱碱性盐,与有机酸生成的盐易溶于水相而被去除。
将调味液的风味成分(表3)与洋河天之蓝白酒[3]对比,调味液的风味酯类总量明显高于白酒,关键风味酯类丁酸乙酯(105.08 mg/L)、辛酸乙酯(232.97 mg/L)的含量远高于白酒,己酸乙酯(1 350.47 mg/L)、戊酸乙酯(26.83 mg/L)的含量和白酒接近,乙酸乙酯(30.72 mg/L)的含量略低于白酒,这与酯类极性对分配系数影响的结果一致,有机酸几乎未检出,调味液具有较强的菠萝型、水蜜桃型等甜果香气而无腐臭、恶臭等不愉快的气味[15-17],可以弥补酸化基酒中酯类风味物质的不足,以提高酒的风味。
表3 酯化液、萃取液和调味液风味化合物的质量浓度 单位:mg/L
Table 3 The concentration of flavor compound in the esterification liquid, extract liquor and the blending liquid

化合物名称酯化液萃取液调味液化合物名称酯化液萃取液调味液酯类酚类己酸乙酯220.64851.911 350.472,4-二叔丁基苯酚0.120.230.37乙酸橙花酯41.00702.051 003.794-甲基苯酚0.0040.0280.0443-苯丙酸乙酯4.35133.92214.97对乙基苯酚0.0810.470.75苯乙酸乙酯35.4382.71132.774-甲基愈创木酚0.160.641.03乳酸乙酯21.55160.98258.41酚类总计0.3641.3682.194辛酸乙酯40.68139.7232.97醇类化合物乙酸芳樟酯29.74113.01182.49二乙二醇1.6118.2629.31丁酸乙酯17.3964.82105.08正丁醇0.625.739.20十六酸乙酯0.4244.0670.73正己醇0.492.764.44庚酸乙酯0.3624.9340.02异戊醇0.352.073.32丙酸香叶酯0.3423.9438.43香叶醇0.331.352.17亚油酸乙酯0.3622.8936.74芳樟醇0.150.901.45乙酸乙酯5.5616.2330.72苯乙醇0.120.661.06戊酸乙酯4.7616.1126.831-戊醇0.010.070.10反油酸乙酯0.2110.0916.2醇类总计3.6831.8151.06乙酸香茅酯0.347.9912.83醛类异戊酸乙酯0.144.286.47醛类总计1.70未检出未检出2-甲基丁酸乙酯0.313.776.05有机酸乙酸正己酯0.532.614.18有机酸总计413.95未检出未检出乙酸异戊酯0.341.672.68酯类总计424.452 427.673 772.83
3 结论
本文采用双水相萃取的方法分离提取酯化液中的风味酯类,选用成相迅速、溶解范围广泛的K2HPO4为萃取用盐,研究发现随着无机盐浓度的升高,酯类的相比显著下降,分配系数先增大后趋于不变,萃取率先增大后减小;分配系数与酯类疏水性有关,极性越强分配系数越小。较优的酯类萃取条件为:K2HPO4质量分数为80%,pH值为4.5,乙醇体积分数为20%,在该萃取条件下,5种酯类的萃取率均达到90%~99%,且萃取液中5种酯类的质量浓度是酯化液的3~5倍。萃取液用无水Na2SO4进一步脱水得到调味液,调味液的酯类含量明显高于黄浆水和市售天之蓝白酒,且不含有机酸,适合酸化基酒的勾兑。另外,在双水相萃取过程中,残余蛋白、糖类、淀粉、醛类等含有亲水基团的物质留在水相而去除,达到了除杂的效果;综上所述,本文首次将新型的双水相应用于黄浆水酯化液中酯类的萃取,为黄浆水的实际应用奠定了理论基础,具有一定的价值。
[1] 冯兴垚, 邓杰, 谢军, 等.白酒酿造副产物黄水综合利用现状浅析[J].中国酿造, 2017, 36(2):6-9.
FENG X Y, DENG J, XIE J, et al.Brief analysis on current situation of comprehensive utilization of by-products yellow water from Baijiu-making[J].China Brewing, 2017, 36(2):6-9.
[2] 梁艳玲, 伍彦华, 苏芬芬, 等.大曲酒副产物黄水的综合利用[J].轻工科技, 2016, 32(10):1-2;20.
LIANG Y L, WU Y H, SU F F, et al.Comprehensive utilization of Daqu liquor by-product yellow water[J].Light Industry Science and Technology, 2016, 32(10):1-2;20.
[3] 杨铭, 李亚男, 陈正行, 等.黄浆水酯化液工艺优化及关键风味物质分析[J].食品与发酵工业, 2019,45(12):160-168.
YANG M, LI Y N, CHEN Z X, et al.Optimized esterifying process of yellow water and analysis of key flavor substances[J].Food and Fermentation Industries, 2019,45(12):160-168.
[4] XIA Q, WU C D, HUANG J, et al.Selection and application of potential whole-cell enzymes in the esterification of Huangshui, a by-product formed during Chinese liquor-making[J].Journal of the Institute of Brewing, 2014, 120(1):45-51.
[5] 蒋学剑, 王志强, 汤井立, 等.黄浆水酯化串蒸提升白酒品质的研究[J].酿酒, 2017, 44(2):60-64.
JIANG X J, WANG Z Q, TANG J L, et al.Study on the improvement of liquor quality by esterifying and cross steaming of the yellow water[J].Liquor Making, 2017, 44(2):60-64.
[6] 李安军, 刘国英, 李兰, 等.基于超临界CO2萃取技术提取酿酒黄水中风味物质[J].食品与发酵工业, 2019, 45(2):118-123.
LI A J, LIU G Y, LI L, et al.Study on the extraction of aroma & flavor-producing substances from the by-products in liquor-making by supercritical CO2 extraction technique[J].Food and Fermentation Industries, 2019, 45(2):118-123.
[7] 唐心强, 左风华, 王虹.基于共沸蒸馏从黄水中获取白酒调味品的方法[J].酿酒科技, 2017(7):107-114.
TANG X Q, ZUO F H, WANG H.Obtaining baijiu flavoring from yellow water by azeotropic distillation[J].Liquor-Making Science & Technology, 2017(7):107-114.
[8] 朱开宪, 张华知, 胡春玲.基于超临界CO2萃取技术提取黄水中风味物质的研究[J].山东化工, 2015, 44(22):35-37.
ZHU K X, ZHANG H Z, HU C L.Study on the extraction of aroma & flavor-producing substances from the by-products in liquor-making by supercritical CO2 extraction technique[J].Shandong Chemical Industry, 2015, 44(22):35-37.
[9] PRATIWI A I, YOKOUCHI T, MATSUMOTO M, et al.Extraction of succinic acid by aqueous two-phase system using alcohols/salts and ionic liquids/salts[J].Separation and Purification Technology, 2015, 155:127-132.
[10] XU S G, LAN K Q, LI J M, et al.Separation of lactic acid from synthetic solutions and the mixture directly derived from corn stover by aqueous two phase extraction[J].Separation and Purification Technology, 2018, 204:281-289.
[11] 姚誉阳, 何华, 左朋礼, 等.聚乙二醇-盐双水相系统萃取头孢呋辛酯的研究[J].化学研究与应用, 2013, 25(5):630-635.
YAO Y Y, HE H, ZUO P L, et al.Study on the extraction of cefuroxime axetil using PEG/salt aqueous two-phase system[J].Chemical Research and Application, 2013, 25(5):630-635.
[12] ZHANG X F, TENG G X, ZHANG J.Ethanol/salt aqueous two-phase system based ultrasonically assisted extraction of polysaccharides from Lilium davidiivar.unicolor Salisb:Physicochemical characterization and antiglycation properties[J].Journal of Molecular Liquids, 2018, 256:497-506.
[13] CHENG Z Y, SONG H Y, CAO X L, et al.Simultaneous extraction and purification of polysaccharides from Gentiana scabra Bunge by microwave-assisted ethanol-salt aqueous two-phase system[J].Industrial Crops & Products, 2017, 102:75-87.
[14] LI Y L, LU X J, LUO Q X, et al.Extraction and mechanistic investigation of trace dibutyl phthalate using an ionic liquid aqueous two-phase system[J].New Journal of Chemistry, 2015, 39(8):6 223-6 230.
[15] 范文来, 聂庆庆, 徐岩.洋河绵柔型白酒关键风味成分[J].食品科学, 2013, 34(4):135-139.
FAN W L, NIE Q Q, XU Y.Key aroma compounds of Yanghe supple and mellow aroma style liquors[J].Food Science, 2013, 34(4):135-139.
[16] FAN H Y, FAN W L, XU Y.Characterization of key odorants in chinese chixiang aroma-type liquor by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies[J].Journal of Agricultural and Food Chemistry, 2015, 63(14):3 660-3 668.
[17] GAO W J, FAN W L, XU Y.Characterization of the key odorants in light aroma type chinese liquor by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies[J].Journal of Agricultural and Food Chemistry, 2014, 62(25):5 796-5 804.
[18] 孙丽慧. 微生物发酵制备2,3-丁二醇及其双水相萃取[D].大连:大连理工大学, 2009.
SUN L H.Microbial fermentation and aqueous two-phase extraction of 2,3-butanediol[D].Dalian:Dalian University of Technology, 2009.
[19] 古博华. 络合萃取和双水相萃取法提取发酵液中丁二酸的研究[D].无锡:江南大学, 2014.
GU B H.Study on recovery of succinic acid from fermentation broth using reactive extraction and aqueous two-phase system[D].Wuxi:Jiangnan University, 2014.
[20] 范蕊庆, 李晓瑄.乙醇/磷酸氢二钾双水相体系分离大豆异黄酮[J].现代化工, 2015, 35(1):99-102.
FAN R Q, LI X X.Extraction of soy isoflavone by ethanol/K2HPO4 aqueous two-phase system[J].Modern Chemical Industry, 2015, 35(1):99-102.
[21] ZOSKI C G.Handbook of Electrochemistry[M].Amsterdam:Elsevier Science Ltd,2017.
[22] 裴莹, 孙玉波, 黄斌.双水相萃取分离α-酮戊二酸[J].食品与发酵工业, 2018, 44(12):150-154.
PEI Y, SUN Y B, HUANG B.The extraction and separation of α-ketoglutarate by aqueous two-phase systems[J].Food and Fermentation Industries, 2018, 44(12):150-154.